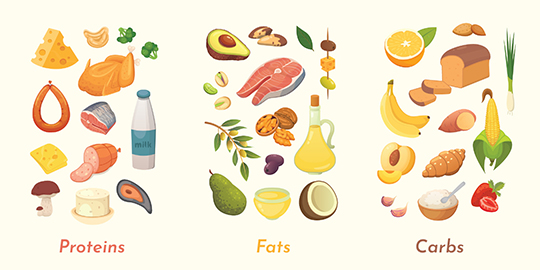CHAPTER 7 Reference Readings
Lesson 2
What is food and what happens to food when we eat it?
What is food?
Do you ever read the Nutrition Facts labels on the packages of foods you eat? Most foods contain at least some of the nutrients that supply the matter and energy that is essential for your body to function. But how are these nutrients similar and different from one another?
In its most basic sense, food is any substance that your body can use as a raw material to sustain its growth, repair it, and provide energy.
The food we eat is made up of hundreds to thousands of different types of molecules. However, humans can obtain the matter and energy they need for life from only three major classes of molecules: proteins, lipids (fats), and carbohydrates. These three classes of molecules are examples of macromolecules. Macromolecules are very large molecules made up of thousands of atoms (macro- means large). Often, macromolecules are made from “subunits” (smaller molecules of the same or similar kind) linked together in long chains. Let’s examine each of these types of molecules in more detail.
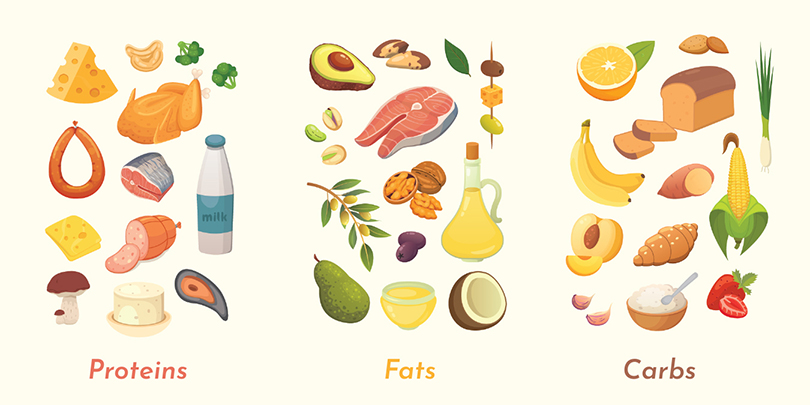
How are the types of macromolecules similar and different?
Proteins
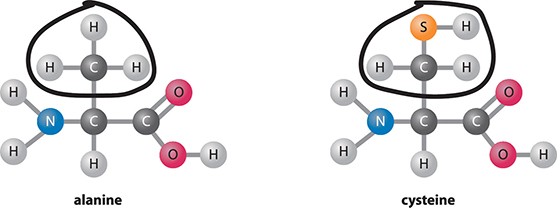
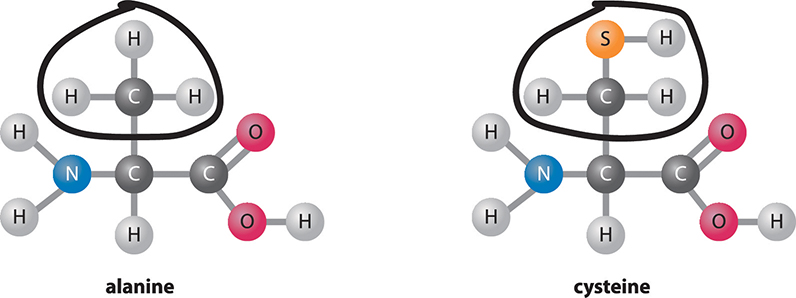
What makes protein in the diet so important? Your body uses the building blocks of protein to make new proteins. These new proteins can have many functions in the body, such as helping with chemical reactions and building structures in a cell. The building blocks of proteins are amino acids. Proteins are made by reacting amino acids together to form a chain. All the organisms on Earth mostly use only 20 different amino acids. You can compare the structures of two different amino acids in the figure below. Proteins in our diets can come from many sources: animal-based foods like meat, milk, and eggs, as well as plant-based foods, like beans, nuts, and seeds. Even fruits, vegetables, and grains are sources of dietary protein, although in lower amounts.
Amino acids. All amino acids have the same basic structure, but each type varies in one region.
Lipids
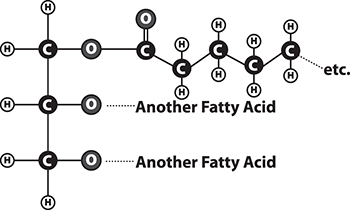
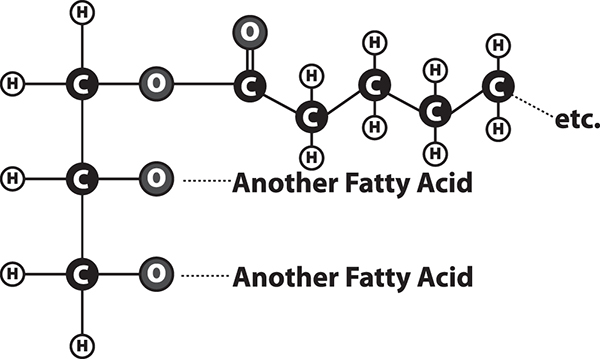
The food we eat can also serve as a source of fat. Look at the structure of a fat molecule in the figure below. Simple fats are made by reacting long chains of carbon and hydrogen called fatty acids and a 3-carbon molecule called glycerol. Fats are part of a larger group of molecules called lipids. Lipids do not dissolve easily in water. Lipids are important nutritionally for making hormones and cell membranes and as a fuel to obtain energy. Most of the fats in our diet come from animal-based fats in meat, eggs, and dairy products, or from vegetable oils, nuts, and seeds. Fruits and vegetables usually contain only small amounts of fats, with a few exceptions like olives and avocados.
Carbohydrates
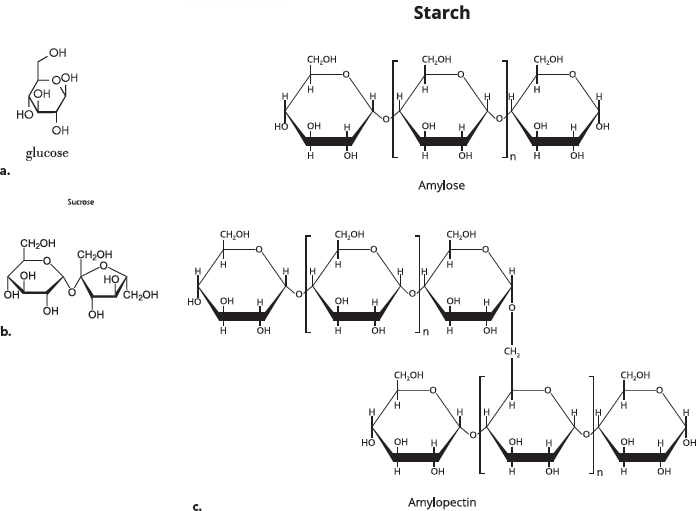
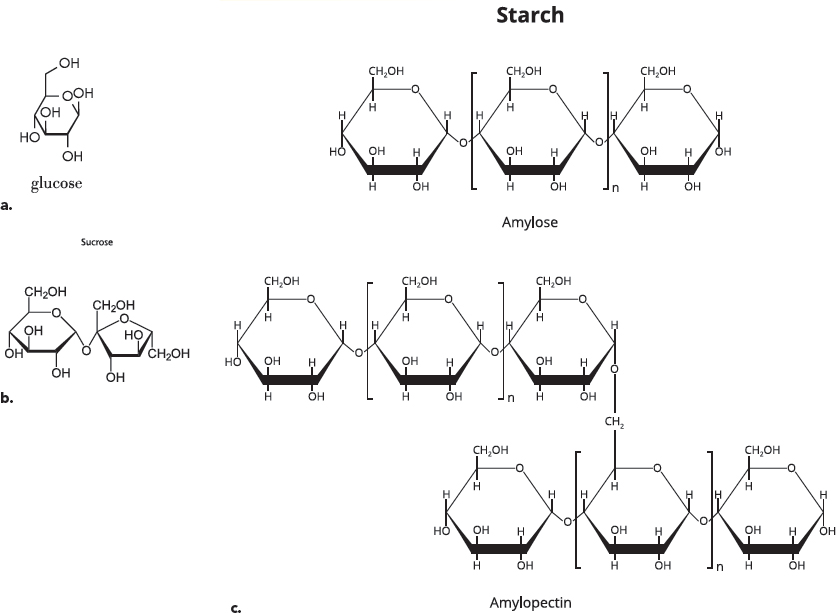
A third type of important organic molecule is carbohydrates. The name “carbohydrate” tells you something about the elements in these molecules. “Carbo-” refers to carbon, and “hydrate” refers to water, which contains oxygen and hydrogen. The building blocks of carbohydrates are single-unit sugars. Glucose, which is the sugar that travels in your blood, is an example of a single-unit sugar. It is the preferred energy source for most cells. Most carbohydrates in our diets come from plant-based foods, like fruits and grains, or from sugars that are derived from plants (like cane sugar). Plants contain very large carbohydrate molecules called starch and cellulose (also called polysaccharides).
Carbohydrates. (a) The simple sugar glucose is the preferred energy source of many cells. (b) Sucrose (table sugar) is formed when two different simple sugars, glucose and fructose, react. (c) Starch is a large carbohydrate made from hundreds of glucose sugars. Two forms of starch, amylose and amylopectin, are shown. Some large carbohydrates can be broken down in our bodies into simple sugars, and others—like fiber—cannot.
Following food molecules in the body
To find out where the proteins, lipids, and carbohydrates in our food go after we eat them, it would be helpful to follow them into the different organs and tissues of our body. To do this, we need a way to tag or label the macromolecules in food so we can distinguish them from the proteins, lipids, and carbohydrates already in our bodies. Scientists do this by using uncommon isotopes (variants) of carbon and other atoms.
For example, most carbon atoms have a molecular weight of 12. A carbon atom like this is called “carbon-12.” Scientists can produce carbon-14 atoms; these atoms have a molecular weight of 14. Thus, carbon-12 and carbon-14 atoms can be distinguished from each other. And conveniently, isotopes will behave the same way in chemical reactions as regular atoms do. Scientists can make food with proteins, lipids, or carbohydrates using carbon-14 atoms. Then they can follow these atoms to find out where they end up in our bodies. Nitrogen atoms can be labeled in a similar way to follow protein molecules through our bodies.
Using model organisms
To find out where the molecules in food go, we could remove and test the organs and tissues within the body (as well as testing waste products from the body). It would be unethical, however, to sacrifice healthy people to figure this out! Remember that when we faced a similar issue in unit 2, we learned that scientists often use model organisms to test their ideas. In this context, we would select organisms that eat and digest the same macromolecules found in human food. Fortunately, many animals eat food similar to human food.
Using isotopes and model organisms like fish and rats, scientists were able to trace where atoms in food ended up. They found that nearly half of the labeled amino acids in food are found in waste products (waste that leaves via the digestive tract as well as CO2 that is breathed out), but the rest is found in proteins throughout the body.
Reference
Adapted from A Human Approach, 5th Edition, Chapter 7, pp 353, 360–364.
Lesson 3
How does matter from food become
part of our bodies?
Born to eat
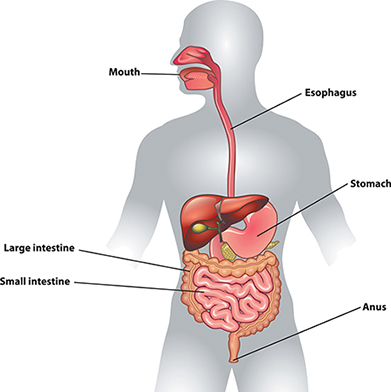
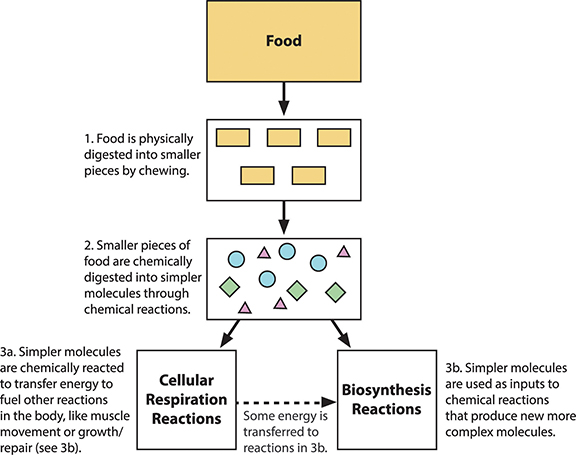
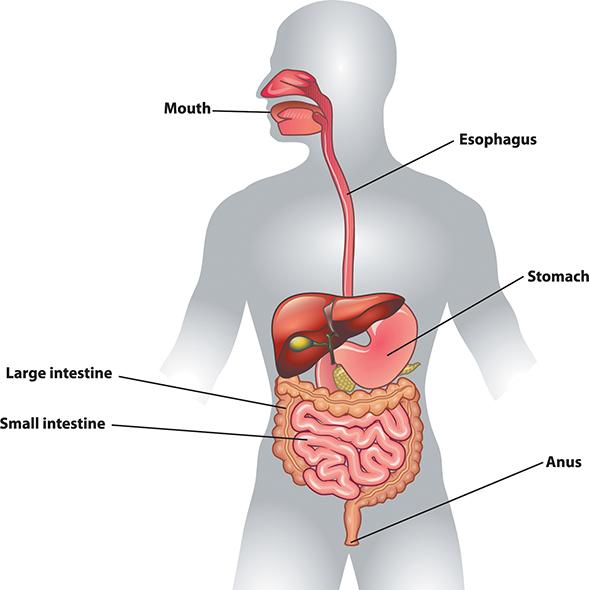
Once we have eaten food, our bodies must break it into smaller pieces that can be absorbed and used by cells. This is the process of digestion. The first stage of the process is physical digestion, which is the breakup of large pieces of food into smaller ones. In humans, it involves chewing. Additional physical digestion occurs in the stomach as food churns and mixes with digestive fluids. The stomach stretches during this process and makes you feel full. The food leaves the stomach as very small bits that travel to the small intestine.
The human digestive system is composed of the mouth, esophagus, stomach, small intestine, large intestine, and anus. The process of breaking down food occurs mostly in the stomach and small intestine. The other organs in the diagram are accessory organs for the digestive system.
The mechanical breakdown of food in the mouth and stomach is only the first stage of digestion. No matter how finely we chew bits of steak, the protein molecules remain intact. If the body is to use the proteins, it must break them down into subunits: amino acids. Similarly, chewed bread still has its carbohydrates intact, mainly in the form of long starch molecules. The body must break down starch to sugars to use it.
The final digestion of the complex food molecules happens in the small intestine in a process called chemical digestion. Chemical reactions break the bonds of the large molecules that make up food and rearrange their atoms to form smaller molecules. Digestive enzymes enable these reactions that break down complex molecules to simpler molecules.
The end products of the digestion of proteins are amino acids; for carbohydrates it’s simple sugars. Cells in the villi that line the small intestine absorb these small molecules through their cell membranes. From there, the small molecules move into capillaries (small blood vessels) associated with the villi. The blood moves the small molecules to all the cells in the body. Once inside the cells, the molecules can be reassembled into the specific, larger molecules that each type of cell needs to support the organism’s growth and repair through a chemical process called biosynthesis.
Biosynthesis
The raw materials that result from digestion, such as amino acids and sugars, serve as building blocks in the synthesis of various body structures. Biosynthesis reactions use these smaller, less complex biomolecules to form larger, more complex molecules that become part of an organism’s body. Examples of biosynthesis reactions are the formation of starch from glucose, and of protein from amino acids. Biosynthesis enables organisms to grow and maintain their structure.
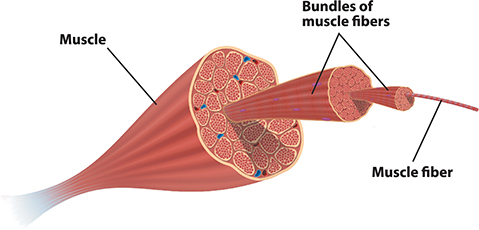
Consider the structure of muscle tissue. Muscles contain fibers that give the tissue strength and provide the mechanism for movement (contraction). The fibers are made of two important proteins: myosin and actin. Muscles also contain enzymes that help repair damaged tissue following exercise. To build new myosin, actin, or enzyme molecules in muscles the body chemically links amino acids together. In unit 2 you learned that genetic information in DNA helps cells put together amino acids in the correct sequence to make functional proteins like these. In this unit we are figuring out ideas related to the source of amino acids and other molecules that become part of cell structures.
Essential amino acids
Humans obtain needed protein from a variety of sources. This variety is necessary to provide all of the different amino acids required for good health. Different proteins require different combinations of amino acids. You need a complete set of amino acids in your body to be able to make all the proteins used by your cells to function. If needed, the human body can synthesize all but nine of the 20 amino acids used to make proteins. These nine must be obtained from food and are sometimes called essential amino acids—it is essential to get them through our diets so that we can assemble all the different kinds of proteins our bodies need.
Food from animal sources (meat, milk, eggs) provide all nine of the essential amino acids for the human diet, as do a few plant sources, like soybeans. Other plant-based foods, like grains, nuts, and seeds, are good sources of protein as well. But most plant-based foods are low in one or more of the essential amino acids. It is possible, however, to obtain all essential amino acids by combining different plant-based foods in the diet. For example, combining legumes (beans, peas, and peanuts) with grains or nuts in a meal can provide all the essential amino acids. This is why peanut butter sandwiches (see image below) are quite nutritious for us (as long as one does not have a peanut allergy). You do not need to get all nine essential amino acids at every meal, but it is a good idea to eat a variety of protein sources over the day.
A peanut butter sandwich. The amino acids in the peanuts complement the amino acids in the bread grains in order to provide the full set of amino acid types needed by our bodies.

Cellulose and lactose: Digestible or not?
Some molecules found in food are not digested by all or by some humans. Cellulose is an example of a large carbohydrate that cannot be digested by any humans. Found in the cell walls of green plants, it is the most abundant macromolecule on Earth. Like starch, cellulose is composed of thousands of glucose subunits linked together. Unlike starch, the bonds that link the glucose subunits in cellulose are different from those that link the same subunits in starch. Humans have enzymes that can break the bonds in starch, but they do not have any enzymes capable of breaking the bonds in cellulose. Cellulose passes through the digestive system and out of the body in feces. Although we do not get any smaller molecules from breaking down cellulose, it is important to have in our diets as fiber that keeps food moving through the digestive system. Cows and other ruminants eat grass because their digestive systems include microorganisms that can break cellulose down into its glucose subunits. Termites also have microorganisms in their guts that break down cellulose, allowing it to be food for them (and also explaining why having termites in your house is a bad thing!).

Lactose is a simple sugar found in the milk of mammals. Lactose is a disaccharide (two-unit sugar molecule) made of a glucose molecule bonded to another similar sugar molecule. The babies and young of mammals make an enzyme called lactase that can break lactose into its two sugar subunits. However, the ability to make this enzyme decreases as individuals age. Most human adults make little or no lactase, although about a third of adults continue to make this enzyme. These people can drink milk and consume other dairy-based foods without problems because they can digest the lactose in it. But for people who do not make lactase, the lactose in milk passes through the small intestine into the large intestine. Bacteria in the large intestine then break down the lactose, producing gas that causes cramps, diarrhea, and general discomfort for their human host.

Reference
Adapted from A Human Approach, 5th Edition, Chapter 7, pp 353, 361–362, 375–378, 380, 433–435 and An Inquiry Approach, Chapter 1, p. 47.
Lesson 4
Why do some atoms from food leave our bodies?
Previously, we saw that a lot of the atoms in food molecules leave our bodies in waste products. We saw that the atoms that do not leave are found in proteins throughout our bodies. Later, we learned how the atoms from food molecules are rearranged into the molecules needed by our bodies in biosynthesis reactions. We also noted that biosynthesis reactions frequently require an input of energy. Where do our bodies get this energy? Do the atoms that leave our bodies give us any hints about how food supplies our energy needs?
Energy associated with molecules
All molecules, including the carbohydrates, proteins, and fats that make up our food, have energy associated with them. The amount of this energy depends in part on the number, types, and arrangement of atoms that compose the molecules. Although all of these types of molecules can be used as a source of energy for our activities, the body uses mostly carbohydrates for this. Glucose is the most direct source of energy for the body.
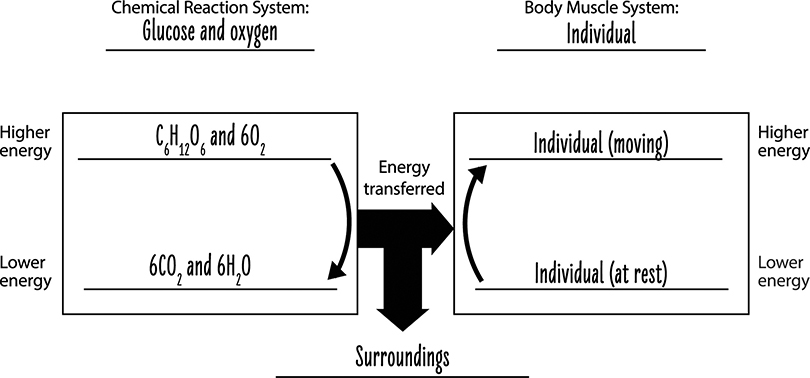
In the cells of our bodies, glucose and oxygen react in a series of chemical reactions that ultimately produce carbon dioxide and water. The name of this series of reactions is cellular respiration. In these reactions, some of the energy associated with the glucose and oxygen molecules is transferred to energy your body uses to grow, repair itself, move, think, and engage in all the other activities of daily living. The remaining energy is associated with the carbon dioxide and water products of the reactions. These molecules are waste products that leave our bodies. In other words, the atoms from food that leave our bodies are products of chemical reactions that provide energy for biosynthesis, movement, thinking, and all of the body’s activities.
Cellular respiration
Overall, the reactions of cellular respiration can be summarized as follows:
Cellular respiration
Glucose + oxygen → carbon dioxide + water
C6H12O6 + 6O2 + 6CO2 + 6H2O
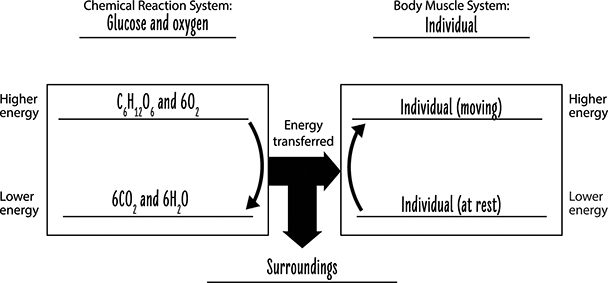
As a system of molecules, glucose and oxygen molecules have a certain amount of energy. Once these molecules react, some of the energy is transferred to other systems that can be used to move muscles as well as provide the energy for other chemical reactions in the body. As described above, some of the energy from the glucose and oxygen system of molecules is transferred to the system of carbon dioxide and water product molecules. In addition, some of the energy from the system of glucose and oxygen molecules is released into the surrounding environment as heat. This is why your body gets warmer when you exercise.
Your body can also use other carbon-based molecules as fuel during cellular respiration. The process is slightly different depending on the type of large carbon-based molecules that react with oxygen at the start, but ultimately the purpose of the reaction is to obtain energy and the main matter products from the reaction are the same.
We can summarize the cellular respiration process by tracing the changes to matter and energy (see the figure below). The atoms in the chemical reaction system of glucose and oxygen are rearranged, providing a net energy gain to our body system. This energy can be used by cells, although some energy is dissipated to the surrounding area as heat. Some of the energy in the glucose and oxygen molecules remain in the chemical reaction system as carbon dioxide and water molecules. There is a lower amount of energy associated with these molecules than with the glucose and oxygen molecules.
Calories
The energy needs of our bodies are met by the food we eat. The energy in food is measured in Calories. A calorie (with a lowercase c) is the amount of energy required to raise the temperature of 1 gram of water by 1 ºC (degree Celsius). One kilocalorie is 1,000 calories. The dietary Calorie (with an uppercase C) is the same as 1 kilocalorie.
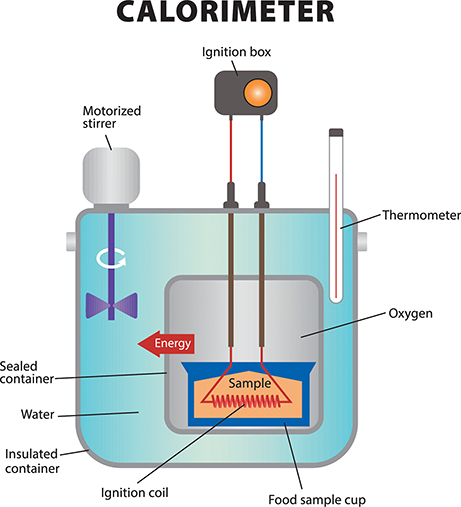
How much energy is available from various foods? Scientists estimate the amount of energy available in food samples using a device called a calorimeter (see the figure below). This device measures the amount of heat generated by the combustion, or burning, of the molecules in a food sample. Thus, using a calorimeter, scientists can calculate the amount of energy contained in the foods.
Food samples are placed in the calorimeter, which is then sealed to prevent heat from the combusting food from escaping to the surroundings. This improves the accuracy of measuring the Calories in food samples.
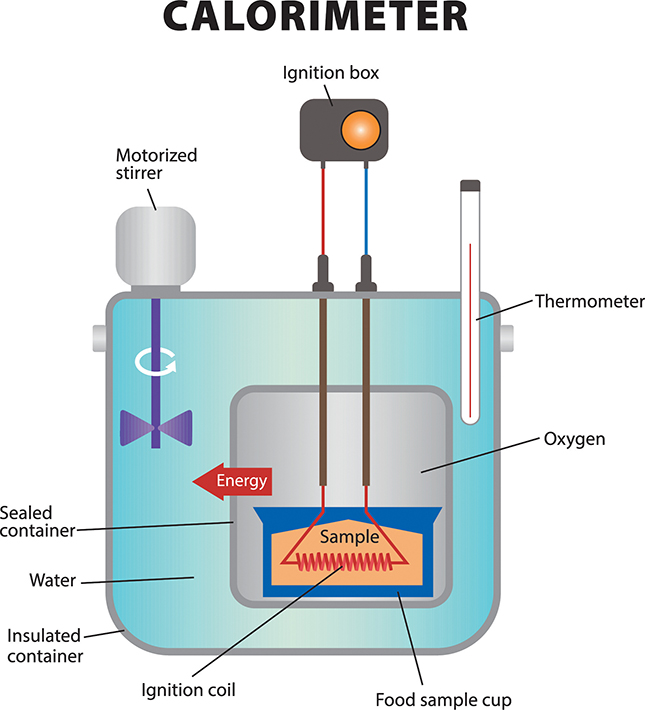
The table below shows the Calories from several food examples. The exact amount your body gets, however, can be tricky to determine. For example, it takes energy to chew and digest the foods. In general, soft foods take less energy to digest than raw foods—consider the difference between eating a raw carrot stick and a cooked carrot. We can usually digest processed foods, whether prepared before they get to the grocery store or in your own kitchen, more completely than raw foods and thus we obtain more of the available Calories from them. Finally, using food to prepare meals may add Calories. If you fry chicken, you are adding Calories from the oil you use to your meal.
Food |
Calories |
|---|---|
Medium banana |
105 |
3 oz. chicken breast |
142 |
8-inch flour tortilla |
140 |
1 tablespoon olive oil |
119 |
2 tablespoons peanut butter |
180 |
12 oz. soda (cola) |
145 |
3.5 oz. tofu |
83 |
The number of Calories found in various foods.
Our bodies use food for energy and biosynthesis
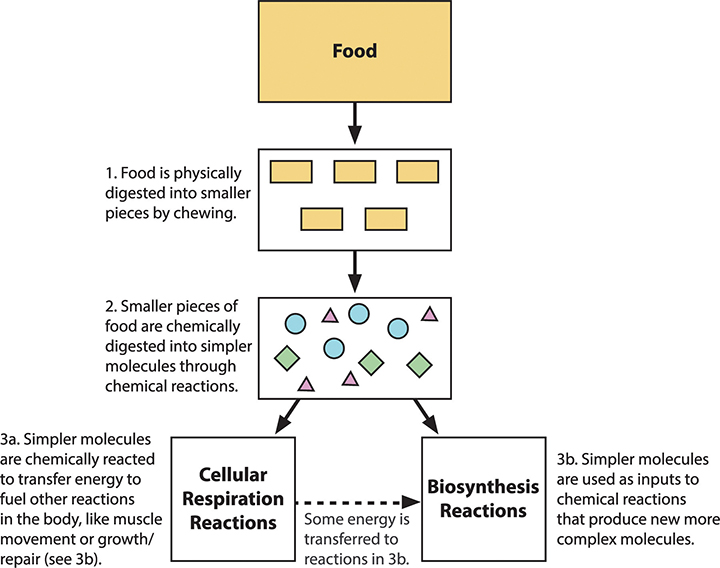
The figure below summarizes what happens to food after we eat. When we eat food, large food molecules are broken down into small molecules that can pass into our bloodstream where they are delivered to all the cells of our body (steps 1 and 2). Small molecules like glucose react with oxygen to form carbon dioxide and water. This process of cellular respiration releases energy that our bodies use to move, build body structures, and stay warm. The carbon dioxide and water molecules pass out of our bodies as waste products (step 3a). Other small molecules like amino acids are used by our cells in biosynthesis reactions to build the specific proteins they need (step 3b).
Reference
Adapted from BSCS Biology: A Human Approach, 5th Edition, Chapter 8, pp 428–429; 433–435.