3.4 Drug Ingredients and Sources
Within a wide range of complex drugs and supplements, there is also a wide range of ingredients. Some are key therapeutic ingredients, and others support the actions, stability, or administration of the key ingredients. The active ingredients are the biochemically active components that exert the desired effects—for example, eradicating bacteria or viruses, lowering blood pressure or cholesterol, or controlling heart rates.
Most medications contain one or more active ingredients as well as several inactive or inert supporting ingredients. An inert ingredient has little or no physiological effect. Common inert ingredients include those that are needed to stabilize the tablet, capsule, or liquid formulation; those that provide the raw material for many topical creams and ointments; those that ensure sterility of injectable products; or those masking the unpleasant taste of some unpleasant oral medications for pediatric patients. These inert ingredients, such as coloring agents or preservatives, can sometimes cause an allergic reaction in a sensitive individual, which is why they must be listed. They are also sometimes called inactive ingredients.
Drug ingredients are derived from various sources and can be classified as natural, synthetic (created artificially), synthesized (created artificially but in imitation of naturally occurring substances), semisynthetic (containing both natural and synthetic components), and biologically engineered (containing genetic materials). Drugs made from natural agents that people are allergic to must be identified so that patients with allergies are not given these drugs.
Natural Sources
Many drugs are derived from naturally occurring biological products, such as single-celled organisms, plants, animals and humans, or minerals. In addition to penicillin and insulin, many other modern-day drugs are derived from natural sources. Even substances from viper venom have been used in medications to fight high blood pressure. Many drugs are from rainforest plant extracts, and PharmaMar, a Spanish drug company, has found almost 100 antitumor compounds from ocean life. Cultures of natural organisms can be grown in laboratories. Some of the natural sources of drugs and their benefits to treatment of diseases are included below:
 Pharm Fact
Pharm Fact
Over 600,000 crabs are captured each spring to donate 30% of their blue blood. It is used to reveal and isolate certain bacteria and help clot hemophiliac blood. Sadly, the crab populations are declining, with 10%–30% lost from blood harvesting.

The blue blood of a horseshoe crab is so valuable to medicine that crabs are captured so that a small bit of their blood can be donated before they are sent back to the sea.
Taxol interferes with growth capabilities of cancer cells (particularly breast cancer) and comes from the bark of the Pacific yew tree.
Streptomycin, an antibiotic used to treat tuberculosis, is from the bacterium Streptomyces griseus.
Vincristine, a drug that inhibits growth of leukemia and other cancer cells, comes from the periwinkle plant in the rainforests of Madagascar.
Limulus amebocyte lysate is a compound from the horseshoe crab’s blue blood that causes clots in the presence of bacterial toxins. It is used to test drugs, vaccines, and surgical implants to ensure that they are germ-free. It also helps the blood of hemophiliacs, whose blood does not clot.
Armour Thyroid, a medication for poorly functioning thyroids, is an extract from desiccated (dried) pig thyroids.
Somatotropin is a human growth hormone from cell lines of the human brain, now often synthesized to imitate the hormone.

Morphine, codeine, paregoric, and heroin trace their source to the opium poppy.
Laboratory Sources
In pharmaceutical manufacturing, many naturally occurring substances have been created or combined with chemical or other ingredients in a laboratory setting. Drugs are classified as synthetic (chemical ingredients), synthesized (chemical ingredients made to imitate a naturally occurring agent), semisynthetic (chemical and natural ingredients together), and biogenetically engineered.
 IN THE REAL WORLD
IN THE REAL WORLD
Synthetic and synthesized drugs have found their way into the illegal drug market. “Designer drugs” are produced in home chemistry laboratories by modifying the chemical structure of existing drugs.
For example, a semisynthetic version of marijuana exerts a similar an effect similar to the natural product but produces more intense hallucinations. Methamphetamine, also known as “meth” or “speed,” is another example of an illegally synthesized drug. Because meth is produced from common decongestants made from pseudoephedrine and ephedrine, community pharmacies are required by law to place restrictions on the sale of products containing this key ingredient. (This is discussed in more detail in Chapter 9.)
Innocent-sounding street names of some dangerous designer drugs are spice, K2, bath salts, plant food, incense, fake weed, and Yucatan fire.
Synthetic Drugs
A synthetic drug is a drug that has been created in the laboratory from a series of chemical reactions to produce a specific pharmacological effect. Phenobarbital—a drug prescribed for seizure, nerve, or headache disorders—is an example of a synthetic chemical compound.
Synthesized Drugs
Ephedrine and aspirin are considered synthesized drugs, or laboratory drugs developed to mimic the pharmacological action of the naturally occurring sources—the Chinese herb ma huang and the white willow (weeping willow) tree, respectively.
Semisynthetic Drugs
Semisynthetic drugs are natural substances combined with laboratory substances designed to (1) improve the efficacy of the natural product; (2) reduce its side effects; (3) overcome medicinal obstacles; or (4) broaden the scope of its uses. Many current antibiotics, such as amoxicillin, are modifications of existing natural drugs, such as penicillin. These antibiotics are more effective against different strains of bacteria or bacteria that have developed resistance to the natural product.
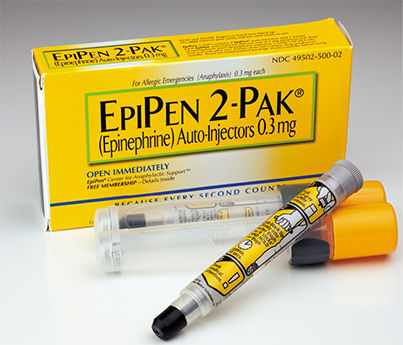
EpiPens contain epinephrine hydrochloride, a lifesaving, injectable synthesized drug used to mimic the actions of the hormone adrenaline for responding to severe allergic reactions, such as from bee stings. EpiPens are often dispensed in packages of two.
Biogenetically Engineered Drugs
Biogenetically engineered drugs are derived from the use of biotechnology to produce unique drugs with specific therapeutic effects, such as the slowing of Alzheimer’s disease. Humulin is an artificial, slow-acting hormone—structurally identical to human insulin—which was developed with DNA technology applied to non-disease-producing laboratory strains of bacteria. Similarly, Novolin is a synthesized insulin using a special strain of baker’s yeast. Both of these insulins are fast-acting and major improvements on an earlier generation of insulins from animal sources that caused more allergic skin reactions.
 IN THE REAL WORLD
IN THE REAL WORLD
Thalidomide was used in 46 countries to alleviate morning sickness in pregnant women, and was later linked to causing birth defects. It was not approved in the United States because FDA drug inspector Frances Oldham Kelsey and her team were not convinced that it had been adequately tested for this new therapeutic use. They refused to approve the medication. Oldham Kelsey’s courage in standing up for safety and caution against the prevailing thought can inspire technicians to speak up and ask questions of the pharmacists, even asking them to query prescribers if something about a drug or dosage seems out of the ordinary or unsafe.