3.6 Nonprescription Drug Approval Process
Since nonprescription medications and supplements are not considered as potent, potentially dangerous, or addictive as prescription drugs, the FDA does not require as intensive an approval process for them.
Over-the-Counter Drug Approvals
Most OTC drugs are meant for self-limited conditions (conditions that could resolve themselves on their own) that are usually dependent upon self-diagnosis for short-term therapy. Other OTC drugs, such as baby aspirin or allergy medicines, may be used on a long-term basis with physician permission.
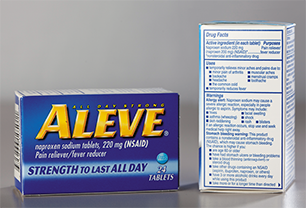
All OTC drugs must include “Drug Facts” labeling for the consumer to safely self-medicate with the product.
To get approval to bring an OTC drug to market, the manufacturer has to submit a new drug application with a specific dose and indication. The FDA must check to see that the drug is safe and effective for use when the labeled directions on the container are followed with regard to dose, frequency, precautions and contraindications, and duration of therapy. After some brand-names prescription medications were proven to have a safe track record and the manufacturer’s patent had nearly expired, the FDA approved an abbreviated new drug application for OTC sales. Examples of such drugs include ibuprofen (Advil), naproxen (Aleve), loratadine (Claritin), cetirizine (Zyrtec), and esomeprazole (Nexium). (See Table 3.1 for common drugs soon to have their patents expire.) When directions are followed, these drugs have been proven relatively safe and effective. When used inappropriately, these drugs can cause harmful side effects, adverse reactions, and interactions with prescription drugs.
Table 3.1 Common Drugs with Patent Expiration Years
Brand Name Drug |
Generic Drug |
Therapeutic Use |
Expected Patent Expiration Year |
|---|---|---|---|
Janumet |
sitagliptin/metformin |
Type 2 diabetes |
2022 |
Revlimid |
lenalidomide |
multiple myeloma |
2022 |
Januvia |
sitagliptin |
Type 2 diabetes |
2022 |
Perforomist |
formoterol |
Asthma and COPD |
2021 |
Pradaxa |
dabigatran |
Prevention of blood clots |
2021 |
Chantix |
varenicline |
Smoking cessation |
2020 |

This image is of a homeopathic medication label.
It is important for OTC drugs to have adequate product labeling, written in easily understood terms, as patients may have no contact with a pharmacist or typed physician instructions. The FDA-required labels contain a prominent “Drug Facts box that lists the active ingredients, purposes, and uses of the product, along with any warnings and directions, including age-appropriate dosing. This “Drug Facts” box allows the consumer to compare active ingredients and assess the benefits and risks of various OTC products. The labels of all OTC drugs must also include a toll-free number to report adverse effects. Also, due to patient allergies and hypersensitivities, the FDA requires manufacturers to list all inert ingredients (as well as the active ingredients) on product package inserts and on bottles of OTC products.
Homeopathic Approvals
The FDA regulates homeopathic therapies like OTC drugs for accurate labeling, but the FDA does not evaluate them for safety or effectiveness. If the manufacturers meet certain conditions and standards set by the USP and the Homeopathic Pharmacopoeia of the United States/Revision Service (HPRS), a compendium of standards and research created by the American Institute of Homeopathy, the FDA allows them to be marketed without agency preapproval.
 Safety Alert
Safety Alert
Most homeopathic medications are available without a prescription, so label directions must be read carefully.
 Put Down Roots
Put Down Roots
Homeopathic comes from the Greek word homeo, meaning “like,” and patheia or pathos, meaning “disease” or “feeling.”
Homeopathic therapy packaging must contain:
an NDC (National Drug Code) number
a list of the active and inactive ingredients (all of which must be on the approved list of the HPRS)
the targeted condition
the number of times an active ingredient has been diluted
directions for use
Dietary Supplements
A dietary supplement is considered a food rather than a drug, so it has different legal oversight. Dietary supplements, including vitamins, minerals, and herbs, can be very helpful for many people to maintain a healthy lifestyle, especially when they are not eating a balanced diet rich in fruits and vegetables. However, consumers should be aware that the quality of many products has been suspect when tested by independent consumer laboratories. Unlike for prescription and OTC drugs, manufacturers of these products are not required to follow USP standards—though voluntary participation is strongly encouraged by the FDA. This means that the manufacturer must label all active and inactive ingredients and cannot make deceptive claims to prevent or cure disease.
Whereas other organizations may provide supplement testing and quality seals, or claim that they do, the USP is the only standard-setting organization for supplements recognized in US federal law. The organization’s standards were acknowledged as the legal standards in the 1994 Dietary Supplement Health and Education Act. Though the FDA does not require manufacturers of supplements to have their products USP certified, it will prosecute them for misbranding if their products display the USP-verified mark or claim to be certified when they are not.

Though many advertisements tried to work vitamins into foods, the FDA did not approve of sales claims for vitamin-enriched donuts.
The USP-verified mark on a dietary supplement label tells consumers that the product quality has been verified under the rigorous USP Dietary Supplement Verification Program. This means that the dietary supplement:
contains the ingredients listed on its label in the declared potency and amounts
does not contain harmful levels of contaminants
will break down and release ingredients into the body within a specified period
has been made according to current US FDA good manufacturing practices
Though dietary supplements are not considered drugs by the FDA, the agency indirectly regulates them. The FDA is allowed to intervene only when proven safety concerns exist. Manufacturers are required to submit data to the FDA on all serious adverse events. The FDA publishes safety alerts and advisories, recalls, outbreaks, and emergency listings online for pharmacists and technicians to review.
Dietary supplements are also regulated by the Federal Trade Commission (FTC). Created in 1914, this agency protects the US consumer from deceptive advertising practices in the marketplace. Though the FDA has primary responsibility for prescription and OTC drug labeling, the FTC regulates the labeling and advertising claims of dietary supplements. A company must be able to substantiate any product claims made in its labeling or advertising. For example, the following are some issues that have been investigated by the FTC:
an herbal supplement that implied it prevented colds
a dietary supplement that claimed to be effective in the treatment of arthritis symptoms
a mineral that said that it improved athletic performance
“miracle” weight-loss product that promised a loss of 15 pounds in 8 weeks
a weight-loss product that did not reveal that it could cause serious increases in blood pressure