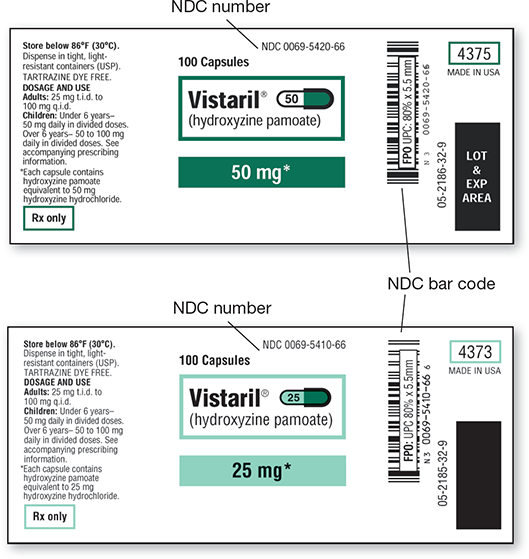
3.7 National Drug Code Number
Under the Drug Listing Act of 1972, discussed in Chapter 2, once a brand name or generic drug receives FDA approval, it is assigned a unique National Drug Code (NDC) number that appears on all drug stock labels as well as on the prescription labels. An NDC number is also assigned to any OTC, homeopathic, or dietary supplement on the market. As shown in Figure 3.3, the 10- to 11-character NDC number is made up of the following parts:
a four- or five-digit labeler code, identifying the manufacturer or distributor of the drug
a three- or four-digit product code, identifying the drug (active ingredient and its dosage form)
a one- or two-digit package code, identifying the packaging size and type
Pharmacists and technicians use the NDC number to check that the correct drug is entered into the computer and dispensed. The pharmacy technician then focuses on the product and package code for filling the prescription, insurance reimbursement, and inventory control.
Figure 3.3 NDC Number and Bar Code
For both of these packages of Vistaril the first four digits of the NDC number (0069) indicate the manufacturer (Pfizer Labs) and the second four the product: 5420 indicates the drug is hydroxyzine pamoate, 50 mg oral capsules; 5410 indicates the same drug, 25 mg oral capsules. The last two numbers identify the packaging size (66), 100 capsules each.