4.3 Prescription Drug Substitutions
As noted in earlier chapters, pharmacists or pharmacy technicians in all states are permitted (or in some cases required) to substitute a lower-cost generic drug in place of a higher-cost brand name drug for a specific indication as long as the generic drug is considered bioequivalent. Unless otherwise noted in this chapter, you will find the brand name drug listed as a proper name with the first letter capitalized and the generic drug/ingredient not capitalized. Sometimes, however, a generic drug will be mentioned with the brand name in parentheses. There are also generic drug brands.
Medications come in different forms. To be a substitution, the drugs must have the same dosage forms.
Pharmaceutically and Therapeutically Equivalent Drug Products
For a specific generic drug to be considered as a bioequivalent for a brand drug by the Food and Drug Administration (FDA), it must be both pharmaceutically and therapeutically equal, though not necessarily identical in appearance.
Pharmaceutically Equivalent
To meet the US Pharmacopeia–National Formulary (USP–NF) standards for strength, quality, purity, and identity, each generic drug is formulated to contain the same amount of active ingredient in the same dosage form as the original, FDA-approved, brand name drug. This makes the two drug products pharmaceutically equivalent. However, these generic drug products may differ in characteristics such as shape, scoring configuration, packaging, inert ingredients (including colorings, flavors, and preservatives), expiration time, and—within certain limits—labeling. A vast majority of all FDA-approved drugs have a pharmaceutically equivalent generic product. A common example is the generic drug lisinopril 20 mg for treating high blood pressure. The dosage unit may look different (i.e., have different markings) than the brands Zestril or Prinivil, but the drug is absorbed in a similar manner.
Therapeutically Equivalent
Each generic drug also needs to be therapeutically equivalent, or provide the same medicinal benefit at the same dosage rates with the same degree of safety under the conditions specified in the labeling as the name brand version. This means that clinical trials show that the generic offers equal results in terms of safety and efficacy, compared to the brand name. The generic depends upon the long track record and earlier clinical studies of the brand name for FDA approval. In the example above, the pharmaceutically equivalent generic of lisinopril should lower blood pressure similarly to the brand product. If a generic drug is both pharmaceutically and therapeutically equivalent, it is considered generally “substitutable” for the brand name product by the technician and pharmacist without prior approval of the physician.
Pharmaceutical Alternative Drug Products
Generic drugs are not available for all brand name drugs (as some patents have not yet expired). Instead, pharmaceutical alternative drug products contain the same active therapeutic ingredients but different salts (for example, metoprolol succinate rather than metoprolol tartrate) or that come in different dosage forms (a tablet rather than a capsule or an immediate-release tablet rather than an extended-release tablet). These pharmaceutical alternatives may be recommended but cannot be substituted without physician approval. Thus, for a prescription of 100 mg of metoprolol succinate (an extended-release formulation), a pharmacy technician cannot substitute 100 mg of metoprolol tartrate (an immediate-release formulation). Although the active ingredients and dose are identical, the salts (succinate versus tartrate) and the release characteristics of the drugs differ. For this reason, this type of substitution is not permitted unless specifically prescribed.
Brand Names Versus Generics
Some physicians do not like to substitute generics even if the medications are considered pharmaceutically and therapeutically equivalent. Just as with brand name drugs, not all patients will respond similarly to a specific generic drug. That is because each patient has a slightly different biological chemistry, and the formulations of inert ingredients affect patients differently. This often occurs with thyroid prescriptions written by an endocrinologist. The prescriber is permitted by law to write “brand medically necessary” on the prescription. Especially on new prescriptions, pharmacy technicians should look for and dispense only the drug specifically written in the prescription. This is a similar directive to a prescriber’s “dispense as written” (DAW).
 Practice Tip
Practice Tip
On each prescription, watch for provider notes. “Brand medically necessary,” “brand necessary,” and “dispense as written” (DAW) all mean the same thing—no substitutions without authorization!
Because brand names are more recognizable, they are often more trusted. The patient may request a brand name drug even if the prescriber did not write “brand medically necessary” or “DAW” on the prescription. The patient’s request would be indicated by writing “DAW2” on the prescription for insurance processing. This generally results in a higher copay, so the technician must make sure that the patient is aware of this. Then the patient should be referred to the pharmacist if they still want the brand name drug.
 Safety Alert
Safety Alert
Always remember that generics and brand names, though substitutable, are not exactly alike. The different formulations may affect patients differently, so when dispensing refills, listen for any patient concerns about substitutions to pass on to the pharmacist.
Conversely, a patient may request a lower-cost generic even though the physician wrote “brand necessary.” A call to the physician’s office will be necessary to approve the generic over the brand name drug.
When refilling a prescription, it is important that the pharmacy technician review the patient profile to see if a brand name or generic product was previously dispensed. For certain medications (such as thyroid hormone replacers or blood thinners), it is not good practice for the patient to keep switching between brand name and generic products even when they are bioequivalent and therapeutically equivalent. Laboratory results often slightly differ though the brand name and generic product are prescribed at the same dose. This is something the technician can explain to the patients, referring them to the pharmacist if necessary.
 IN THE REAL WORLD
IN THE REAL WORLD
The dispensing of generic drugs is a major factor in containing the high cost of pharmaceuticals, with a cost savings of 30% to 80% over brand name counterparts. However, as brands are being developed, costs are rising there too. For instance, in seven months (from October 2013 to April 2014), the cost of the generic asthma medication albuterol sulfate rose from $11 for two tablets to $434 and the antibiotic doxycycline hyclate from an average of $20 per bottle to $1,849! Even so, generic drugs have considerably slowed the rate of cost increases on pharmaceuticals—but it is difficult to tell now by how much. In the same way, biosimilars are projected to save over $5 billion in healthcare costs over the next decade as more expensive biotechnology drugs come off patent.
The FDA Orange Book
A technician or pharmacist is not expected to remember all the pharmaceutical and therapeutic equivalencies or which are legal substitutions in what situations. The pharmacy software will help make these considerations clear and offer alerts. The online FDA Orange Book (officially known as the Approved Drug Products with Therapeutic Equivalence Evaluations)—is the official legal source for drug equivalency information in the United States. The web publication found at: https://PharmPractice7e.ParadigmEducation.com/OrangeBook provides information on generic substitution of drugs that may have many different brand name or generic manufacturer sources. It lists all the drug products the FDA considers to be (or not to be) therapeutically equivalent to other pharmaceutically equivalent products. It covers not only generic substitutions and alternatives, but also brand name alternatives at different dosages.
 Pharm Fact
Pharm Fact
The FDA Orange Book first appeared in October 1980. Since it was near Halloween, the staff chose orange for the cover color, and the nickname stuck. For the biological drug guide, one staff member simply suggested purple, giving rise to the Purple Book.
The Orange Book lists the doses that may be safely substituted. For example, two brand-name antihypertensive drugs for extended (24 hours) blood pressure control are Adalat CC and Procardia XL. They cannot be substituted for each other due to different drug-release characteristics. There are, however, generic manufactured drugs using the active ingredient nifedipine that are compatible with either or both that can be substituted (see Table 4.1). However, each manufacturer’s formulation of salts or other ingredients offers different drug characteristics. Not every generic of the same active ingredient is the same. Some are appropriate as a substitution of one brand formulation and some for another. It depends on the drug formulation and its release characteristics, such as delayed or extended release.
Table 4.1 Generic Drugs Therapeutically Equivalent to Adalat CC and Procardia XL
Generic Drug (Trade) |
Dosage |
Manufacturer |
|---|---|---|
nifedipine (Adalat CC) |
30 mg, 60 mg |
Par |
nifedipine (Adalat CC or Procardia XL) |
30 mg, 60 mg |
Valeant |
nifedipine (Adalat CC or Procardia XL) |
30 mg, 60 mg, 90 mg |
Mylan, Novast, Osmotica, Sun Pharma, TWI, Zydus |
For example, the Par generic formulations of nifedipine in doses of 30 mg and 60 mg can be substituted for Adalat CC. The Mylan, Novast, Osmotica, Sun Pharma, TWI, and Zydus versions can be used for either Adalat CC or Procardia XL. In most cases, the software your pharmacy or drug wholesaler utilizes will cross-reference the FDA-approved generic equivalents of a given brand name drug.
As you learn drug names, it will be easier to remember them if you can remember them in families. The generic names of certain classes of drugs for specific indications often share the same suffix at the end. A great resource list of generic drug name prefixes, roots, and suffixes can be found at https://denalirx.com/drug-prefix-root-and-suffix/. Table 4.2 provides a sample.
Table 4.2 Sample of Common Generic Name Suffixes with Drug Class/Category
Suffix |
Generic Examples |
Class/Category |
|---|---|---|
-artan |
losartan, olmesartan, valsartan |
Angiotensin receptor blocker |
- afil |
sildenafil, tadalafil, verdenafil |
Phosphodiesterase inhibitor |
-cillin |
amoxicillin, ampicillin, nafcillin |
Penicillin derivatives |
-dipine |
amlodipine, nicardipine, nifedipine |
Calcium channel blockers |
-erol |
albuterol, formoterol, salmeterol |
Bronchodilators |
-floxacin |
ciprofloxacin, levofloxacin, moxifloxacin |
Fluoroquinolones |
-lol |
atenolol, metoprolol |
Beta-blockers |
-one |
dexamethasone, methylprednisolone, prednisone |
Corticosteroid |
-prazole |
esomeprazole, lansoprasole, pantoprazole |
Proton pump inhibitors |
-pril |
benazepril, enalapril, lisinopril |
Angiotensin converting enzyme inhibitors |
-statin |
atorvastatin, rosuvastatin, simvastatin |
Statins (HMG-CoA reductase inhibitors) |
-tidine |
famotidine, ranitidine |
Histamine-2 antagonists |
-tropium |
ipratropium, tiotropium |
Anticholinergics |
-zepam |
diazepam, lorazepam, temazepam |
Benzodiazepines |
Biologically Comparable Drug Products
Biological drugs (including genetically engineered drugs) come from a variety of live or once-live sources, including animals, humans, and microorganisms such as yeast, bacteria, and fungi. These drugs are usually expensive injectable drugs used to treat genetic disorders, blood disorders related to cancer or chemotherapy, and inflammatory diseases, such as rheumatoid arthritis. These biological drug products tend to have larger and more complex molecules than most chemical compounds. When a brand-name biological drug is patented and FDA approved, it becomes the biological reference product for all similar biological drug products to come because it is the original drug that went through the extensive clinical trials for safety and effectiveness. A biological drug patent lasts 12 years.
 Pharm Fact
Pharm Fact
As of early 2019, the FDA had approved 19 biosimilar drugs. Different states have different rules. To see a summation of the fall 2018 state rules, go to https://PharmPractice7e.ParadigmEducation.com/NCSL.
Because biological drugs come from living matter or organisms, they cannot be chemically reproduced with exactitude. Therefore a generic version could not be specifically designated as pharmaceutically or therapeutically equivalent. That is why biological drug substitutions are handled differently. Instead of bioequivalent drugs, the FDA has evaluated and approved biosimilar drugs, which are similar though not identical to the parent innovator drugs. Some of these can be tested to be designated as interchangeable biological drugs, which are both biosimilar and have been proven to offer similar therapeutic results. These interchangeables can therefore be legally substituted for the biological reference products in prescriptions.
Biosimilar Drugs
Biosimilar drugs are deemed by the FDA as biologically comparable or sufficiently like the reference products in therapeutic methods. They have been proven to address the same indications with the same pharmaceutical mechanism of action, or precise ways that the active ingredients exert their influence on the body. They must also have the same administration routes, dosage forms, conditions of use, and levels of quality and strength as the original. (See Figure 4.1.)
Figure 4.1 Sample Production Process of a Biological Reference Product
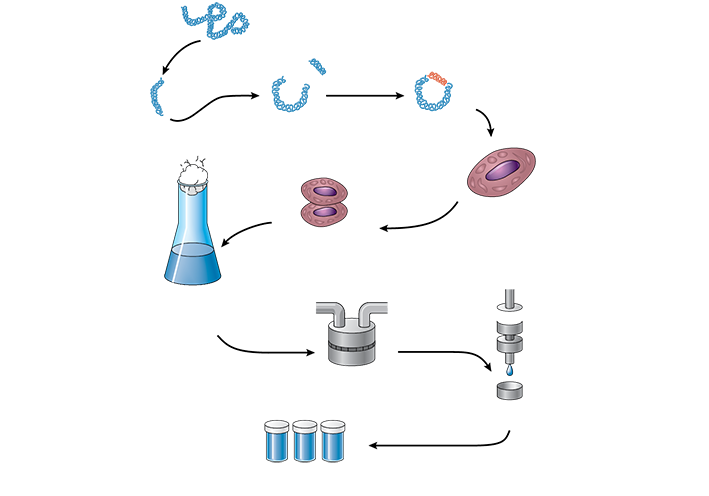
A biosimilar drug must demonstrate no substantial differences in terms of safety, purity, and potency from the original drug, but it may not yet have proven to provide the same clinical effectiveness as the original. Biosimilars that have been FDA-approved can be considered by physicians as alternative drug products for the related biological drug brands. However, the laws governing pharmacy substitution of a biosimilar for a biological brand vary from state to state. Some states allow substitution by the pharmacy as long as the prescriber and patient are notified.
 IN THE REAL WORLD
IN THE REAL WORLD
While pharmacists are allowed to substitute generic drugs for another therapeutically equivalent generic drug in most cases, the same is not always true for biosimilar drugs. While the FDA carries out the scientific review of biosimilar products, it does not determine whether a biosimilar can be substituted for the innovator product. The FDA has deemed this decision up to individual state boards of pharmacy. Some states may permit a pharmacist to substitute a biosimilar product without prescriber authorization. Other states do not allow this change. Check with your state board of pharmacy for biosimilar substitution laws.
Interchangeable Biological Drug Products
To be designated an interchangeable biological drug, a biological drug must be biosimilar to the reference drug and must exert the same therapeutic effect.
The pharmaceutical manufacturer must present scientific data from clinical trials to the FDA indicating that the innovative interchangeable drug product can deliver similar results with the same effectiveness and safety as the reference drug. In addition, there can be no greater risk posed in switching from the reference drug to the interchangeable product.
If all these criteria are met for the FDA to approve the biosimilar as an interchangeable biological drug, pharmacists and technicians may be able to legally substitute it for the prescribed biological brand without contacting the physician for approval, as in a generic drug substitution. However, it is up to the discretion of each state whether or not to allow this substitution.
 Practice Tip
Practice Tip
Remember that biological drugs are from live or once-live organisms. Biologically interchangeable and biosimilar drugs are never bioequivalent to the patented formulation, unlike most generic drugs, which are bioequivalent.
The FDA Purple Book
Just as the Orange Book is a reference for therapeutically equivalent drugs, there is also a reference for interchangeable biological drug products. The online reference is published by the FDA and is titled Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations. Since that is quite a mouthful, it is just called the Purple Book. It currently has two FDA lists:
Over 100 licensed biological drug products that specifically include natural substances (that may be alive), such as allergenic extracts, blood and blood components, gene therapy products, human tissues, and cellular products used in transplantation, vaccines, and more
Over 275 licensed biologically engineered (bioengineered) or biotechnical (biotech) drug products, such as enzymes, protein growth enhancers, and antibodies developed as targeted cancer therapies
As biosimilars and interchangeables start to move through the approval processes, they will be included in the Purple Book. The FDA also publishes the Green Book: FDA Approved Animal Drug Products for veterinary-oriented substances. Because some of these animal drugs can be purchased at pharmacies, this is a valuable reference for pharmacists and technicians. Other common pharmaceutical references will be listed at the end of this chapter.