5.8 Drug Delivery Systems
A drug delivery system is an engineered technology used to target the delivery and/or timing of a drug to be most therapeutic. It takes into consideration the absorption, distribution, and elimination of the drug from the body. Drug delivery systems can be part of a design feature of the drug’s ingredients and/or form or a delivery device.
 Pharm Fact
Pharm Fact
Quillichew ER (methylphenidate hydrochloride) is a medication for patients 6 years and above with ADHD. These cherry-flavored chewable tablets are comprised of tiny coated spheres of medication that dissolve at different rates, with 30% immediate release and 70% delayed release. It is a Schedule II drug.
Oral Dosage Forms with Drug Delivery Systems
Oral dosage forms with specialized drug delivery systems include the disintegrating tablet, the sublingual tablet, and the buccal lozenge. These products have modified absorption and distribution phases in order to enhance their therapeutic effects. Other tablets and capsules have been formulated with an enteric coating to delay their release and protect the stomach or extend their release (SR, XR, XL) over a 12- to 24-hour period. These extended-release medications have a less frequent dosing schedule, which improves patient convenience and adherence and, ultimately, provides better disease control.
Delayed-Release Tablets and Capsules
Most tablet and capsule formulations are immediate-release; that is, the medication is designed to be activated or released shortly after the drug is taken. When treating an infection, immediate-release formulations are desirable to initiate a fast onset of action. However, when treating certain conditions, such as hypertension or hyperglycemia, immediate-release formulations would require a long-term, frequent dosage schedule, which may be an inconvenience to patients. For these patients, a medication designed to control blood pressure or blood glucose levels for 24 hours would be preferable.
Many tablets and capsules are available in a confusing alphabet of release formulations: DR, CR, CD, XR, SR, XT, and XL (see Table 5.7). The abbreviations DR (delayed release), CR (controlled release), and CD (controlled delivery) all refer to drug formulations that have some form of delaying the onset of the drug delivery and/or delaying and then incrementally delivering the drug. The abbreviations LA (long action), TR (timed release), SR (sustained release), XT (extended time release) and XL (extended length/release) have some immediate delivery but extend the effects by incrementally releasing more medication or having longer-lasting ingredients.
Table 5.7 Drug Delivery Abbreviations
Abbreviation |
Meaning |
|---|---|
CD |
Controlled delivery |
CR |
Controlled release |
DR |
Delayed release |
LA |
Long acting |
SR |
Sustained release |
TR |
Timed release |
XL |
Extended length/release |
XT |
Extended time release |
Pharmaceutical manufacturers market different release formulations to serve patients but also to extend their existing product lines, lengthen patent protections, and improve bottom-line profits. For example, Depakote—a medication commonly used for seizures—is available as both a DR enteric-coated drug and an ER or extended-release drug. Many other medications are also designated with release formulations. For example, the antihypertensive drug diltiazem and the antidepressant drug bupropion (Wellbutrin) have multiple immediate-release and extended-release products from several drug manufacturers. However, these drugs are not interchangeable, and their inadvertent substitution could lead to toxicity, patient injury, or, possibly, patient death.
 Safety Alert
Safety Alert
Never substitute a different release formula of the same drug without direction from the pharmacist to do so as it may cause great harm to the patient.
The pharmacy technician must be aware of the many versions of common drug products on the pharmacy shelf—enteric-coated, delayed-release, sustained-release, extended-release, and so on. Many medications with special drug release characteristics do not have a generic equivalent.
In light of that, you must exercise caution when filling prescription medications and pay special attention to these initials at the end of medication names to avoid medication errors. (For more information on medication safety, refer to Chapter 14.) Technicians also need to be aware that many of these delayed-release and extended-release formulations come with a higher cost or copayment for consumers, and some are paid for by insurance while others are not. Some of the ways these different delays and extensions have been designed into the drugs have already been mentioned, but they could use a little more explanation.
Enteric-Coated Tablets As mentioned earlier, delayed-release tablets have a special enteric coating that prevents immediate release until after digestion so that the drug is not released in the stomach itself. Because the enteric coating resists breakdown by acidic gastric fluids, the side effects of nausea and stomach upset are theoretically minimized. Enteric-coated tablets (ECTs) include aspirin, naproxen, and potassium chloride—all drugs that can be irritating to the stomach. Unlike an ECT, a sugar coating on a tablet makes the drug more palatable but does not affect or delay the release characteristics of the drug in the bloodstream.
Gradually Releasing Medications These extended-release (XL) formulations begin their work at delivery, but they have mechanisms that allow them to release their active ingredients slowly. The USP defines extended-release as “formulated in such a manner to make the contained medicament available over an extended period of time following ingestion.” Both sustained-release (SR) formulations and controlled-release (CR) formulations belong to this category. For example, SR formulations, such as bupropion SR, allow a less-frequent dosing schedule (two doses, 8 to 12 hours apart) than the immediate-release counterpart (three to four daily doses). CR formulations allow at least a twofold reduction in dosing frequency from that of immediate-release and most SR formulations. For example, Wellbutrin XL should be taken once a day in the morning whereas Wellbutrin SR is taken every 8 to 12 hours.
 Safety Alert
Safety Alert
Beware: a sustained-release (SR) dosage form is not the same as an extended-release (XL) dosage form of the same drug. They are often confused by technicians.
Extended-Release Tablets and Capsules
There are different ways to accomplish extension of drug availability within a system. Several options offer different formulations that attain these extended drug deliveries.
Differing Drug Salts Differing delivery effects can be achieved by altering the drug salts that are mixed with the active ingredients. The oral nitrate tablet isosorbide 30 mg is available as a mononitrate salt (with once-daily dosing) and a dinitrate salt (twice-daily dosing) to prevent chest pain. Another example is the antihypertensive drug metoprolol. It is available as an immediate-release tartrate salt and also as an extended-release succinate salt in doses of 25 mg, 50 mg, and 100 mg. Though the active ingredients are exactly the same, these drugs are not interchangeable—even at identical doses—and the pharmacy technician must take care and precaution to select the correct stock bottle.
Wax Matrix Delivery Systems In wax matrix systems, the drug is embedded in an inner chamber surrounded by a waxy substance, and a portion of the drug is partitioned in the inner core of the tablet by the waxy matrix (material surrounding it) for later release. As the outer drug portion is digested, the inner portion is sustained until the wax substance is dissolved or eroded, at which point the encapsulated drug ingredients are released. The narcotic drug OxyContin is an example of a matrix-controlled release (CR) formulation in which the medication is slowly released over a period of 12 hours.
Ion-Exchange Resin Capsules of hydrocodone/chlorpheniramine (Tussionex), an extended-release (XR) narcotic cough medication, are imbedded in a special substance that encourages slow exchanges of the drug ingredients through a permeable resin coating that diffuses the release of the drug. This drug design permits a gradual release of the active ingredients over 12 hours. In addition to an increase in the duration of action, this formulation has a resin coating that can mask taste, prevent tablet disintegration, and improve the chemical stability of the active ingredient.
Osmotic Pressure Release Formulations The osmotic pressure system uses the power of water to equalize pressure and salinity between the inside and outer regions of the formulation. The tablets have a rigid coating that has microscopic laser-created holes. As the watery fluids from the digestive system push in, they slowly push the medicine out of the tablets into the digestive system, and into the bloodstream. This drug delivery system has several therapeutic uses for a 24-hour drug delivery:
to maintain a constant drug plasma concentration for behavioral control—methylphenidate (Concerta), amphetamine/dextroamphetamine (Adderall XR)
to regulate blood glucose levels—glipizide (Glucotrol XL)
to manage depression—venlafaxine (Effexor XR)
to treat hypertension—nifedipine (Procardia XL)
Other Innovative Extended and Targeted Drug Delivery Systems
Other recent drug delivery systems employ biotechnologic delivery processes to produce long-term therapeutic effects. The transdermal patches have already been described. They are formulated to release their medication slowly over 12 hours, 24 hours, 72 hours, or one week. Drugs can also be injected into a kind of internal pool or reservoir so that the surrounding tissue can gradually absorb the pool. This is called a depot injection. Other innovative systems include targeted drug delivery systems and surgical implants.
Targeted Drug Delivery Systems
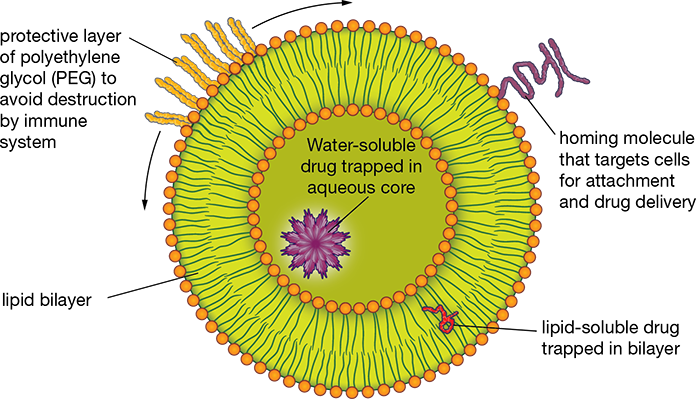
Targeted drug delivery systems deliver the medication directly to the targeted internal body location. This intensifies and prolongs the therapeutic action, as the effects of the medication are not diluted by being dispersed throughout the whole bodily system. The drug is injected directly into a tumor or affected area or “carried” by a specific substance to the correct area. The most common current carrier is a liposome. It is a bubble-like sphere made of organic cell membrane-like material (often of natural fat or lipid material) generally holding a watery fluid sac. The active drug ingredients are inserted into the fluid of the sac (see Figure 5.11). The liposome is covered with a protective coating. Attached to the covering are “homing” molecules designed to be attracted to the target site—for instance, a cancerous site. The liposomes often have a “head” molecule that is attracted to water and a “tail” that is not; this has the effect of naturally propelling the liposome forward. This liposome drug delivery system is commonly formulated for chemotherapeutic drugs, targeting only the cancerous organ.
 Put Down Roots
Put Down Roots
Lipo in Greek means “fat,” and soma means “body,” so liposomes are sometimes known as fat bodies.
Employing this method avoids the adverse effects associated with other chemotherapies, such as decreased blood counts, alopecia (hair loss), and severe GI disturbances that occur when the toxic agents are distributed systemically.
Figure 5.11 Liposome
Using a liposome to deliver drugs to specific cells increases the drug’s effectiveness and reduces toxic side effects.
Internal devices can also target the drug delivery. Vaginal rings and hormonal intrauterine devices are inserted vaginally to prevent pregnancy. These contraceptive devices provide a slow release of hormones when placed in contact with the mucosa of the reproductive tract (so they utilize the transmucosal route of administration).
NuvaRing is an example of a vaginal-ring delivery system used to prevent conception. The ring is two inches in diameter and is made of a flexible plastic that is inserted vaginally once a month. Once in contact with the vaginal mucosa, estrogen and progestin hormones are released and slowly absorbed and distributed into the bloodstream. This steady, consistent release of medication may result in fewer hormonal variations than when using other hormonal methods. NuvaRing is as effective in preventing conception as oral contraceptives. In fact, a patient may be more adherent to therapy with a once-monthly ring rather than the daily ingestion of an oral contraceptive. It is recommended that the contraceptive ring be refrigerated before being dispensed.
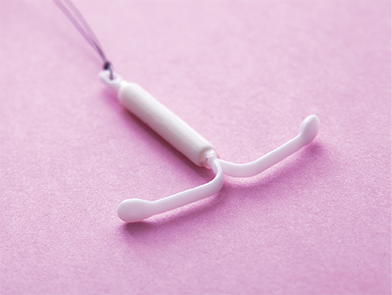
An IUD like this releases a progestin-only hormone within the uterus that prevents conception.
An intrauterine device (IUD) is a t-shaped plastic device that is nonsurgically fitted into the uterus through the vagina by a physician as a drug delivery form to prevent pregnancy. IUDs come wrapped in copper (such as Paragard) or contain a hormonal drug that damages or kills sperm, discouraging conception. Hormonal IUDs contain a progestin-only hormone that is slowly released and localized primarily in the uterus, thus decreasing the risk of long-term adverse effects. This device provides a higher degree of contraceptive protection with a lower failure rate than tablets, patches, and contraceptive vaginal rings.
 Pharm Fact
Pharm Fact
Because IUDs do not have to be changed for 3 to 10 years, it can help with therapy adherence, especially for women who forget to take oral forms. An IUD may also be less expensive. However, it can shift position, which can cause mild to severe difficulties.
 IN THE REAL WORLD
IN THE REAL WORLD
Scientists are always experimenting with more efficient ways to release drugs and have them keep releasing over time. A new form called hydrogels is an injectable gel drug that is pliable and offers extended release of the drug. Researchers see this as ideal for filling a cavity left after the removal of a cancer tumor. The hydrogel form of the chemotherapy could help the area heal by taking up pliable space while slowly releasing cancer-fighting components. The hydrogel form will also work to help stop macular degeneration, a deterioration of the eye that is presently addressed by periodic injections in the eyes. This hydrogel form of drug could cut the injection rate from six to one. This delivery form is still in the stages of experimentation, but you can watch for it to be available soon!
Surgical Implants
Other innovations in drug delivery systems include surgically implanted catheters (thin flexible tubes) and drug pumps, which can provide medication either through the catheter to an external pump or to an internal pump. This has been used, for instance, with pain drugs administered to the spinal cord. Eyes have been implanted with various devices for drug delivery that are made of organic and inorganic materials. Radioactive “seeds” have been implanted in prostates to provide ongoing targeted chemoradiation to the cancerous area. Because these implants and approaches are not commonly seen by pharmacy technicians, they will not be described, but it is good to be aware of how vast and innovative the full field of drug delivery systems is.