7.8 Technician and Accuracy Checks
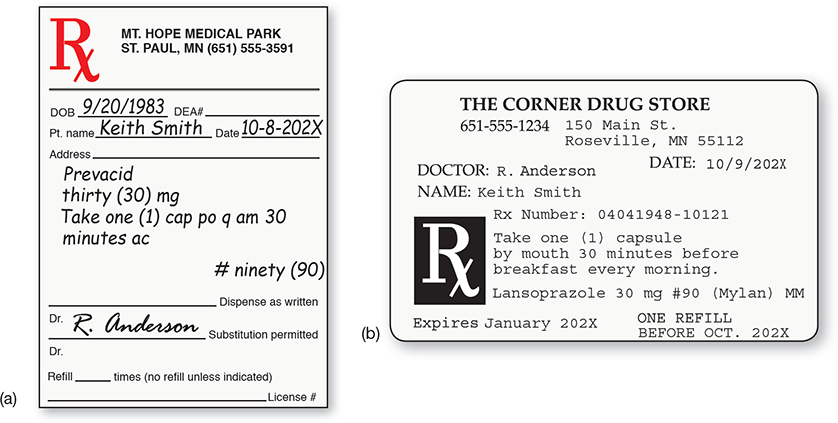
Throughout the filling process, the technician must continue to check the prescription, the printed label, the NDC provided by the pharmacy software, and the stock drug container. Since the prescribed brand is different from the filled generic, the prescription and the label will not match for the drug name, and the NDC of the prescribed medication will not appear on either. Instead, the pharmacy software will provide the new NDC number and communicate it to the bar code scanner. Tech-nicians must check both. Though a generic drug may have been substituted, the patient instructions on the label must read exactly as indicated on the original prescription’s signa (see Figure 7.12).
 Safety Alert
Safety Alert
Exercise caution when selecting insulin from the refrigerator. Insulin mix-ups are a common medication error.
Figure 7.12 Prescription and Label Comparison
The original prescription (a) contains precise instructions, or signa, for the patient that must appear on the label (b).
 IN THE REAL WORLD
IN THE REAL WORLD
If prescriptions filled on a given day in the United States were only 99% correct, then the average pharmacy filling 200 prescriptions daily would have 2 incorrect prescriptions each day. This is unacceptable. According to a study in the Journal of the American Medical Association, more than 100,000 Americans die each year of adverse drug reactions. This means that harmful drug responses are the sixth leading cause of death in the United States. (Some estimates are higher, making medication errors the third leading cause of death.)
Part of the problem is consumer expectations for quick prescription filling, but also responsible are long hours without enough breaks for technicians and pharmacists, insufficient training and certification for technicians, understaffing, multitasking, and distractions.
The Minnesota Board of Pharmacy began taking public comments in 2015 about a new law for mandatory breaks for pharmacy personnel to prevent fatigue-induced medication errors. One pharmacist wrote about being distracted due to feeling lightheaded and shaky from hunger as she was given no breaks to eat. Pharmacies that have been cutting staff and lengthening shifts have been magnifying the problem. Various pharmacy boards are starting to address these issues.
Before completion, a final bar code scan of the stock NDC and comparison with the label is an essential safety check to avoid accidentally having selected a similar looking but incorrect bottle. For instance, selecting the wrong insulin from the refrigerator is one of the most common medication errors. As noted earlier, the names of several types of insulin manufactured by Eli Lilly are quite similar: Humulin R, Humulin N, Humalog, Humalog mix 50/50, Humulin mix 70/30, and Humalog mix 75/25. Some types of insulin are available in 10 mL vials; other insulin is administered through the use of prefilled pens. It is easy to inadvertently select the incorrect insulin from the refrigerator, but bar code scanning will detect the error.

These bottles show the same drug, manufacturer, and package size, but the dosages are different. Scanning the NDC bar code helps ensure that the technician selects the right bottle, even when feeling rushed.
Although speed is important, 100% accuracy is most important—a life-or-death matter, many times. Precise work habits need to become ingrained in technicians, including carefully avoiding drug retrieval based just on drug manufacturer label design (i.e., two different drugs from the same generic manufacturer stored in close proximity to one another) but instead utilizing bar code technology and carefully reading and comparing the stock drug label with prescriptions and printed labels.
You must integrate three separate NDC checks as habits to prevent errors: (1) when the product is initially being pulled from the inventory shelf; (2) at the time of preparation; and (3) when the stock product is returned to the shelf. Do these same checks when filling any automatic dispensing machines. (For more information on medication safety practices, see Chapter 14.)