12.6 The Cleanroom
According to USP <797>, CSPs need to be compounded in a cleanroom, which has highly controlled air quality to ensure an environment as germ free as possible with no dust and low levels of particulates in the air to avoid contamination. The standard compounding cleanroom is separated into two separate rooms: a preparatory anteroom and a buffer room that provides space for the sterile compounding equipment (which will be described in depth later in the chapter).
Globally, air quality is measured by the fractional number (in micrometers) of particles (contaminants) that can be found per cubic meter (m3) of air. This classification system is from the International Organization for Standardization (ISO). Particles in the air are counted with a light-scattering device that tallies the particles of different sizes in a cubic meter of compressed air. The lower the fractional number of particles, the lower the ISO class number and the better the air quality. Sterile compounding needs to be done at ISO Class 5 or less, as opposed to typical indoor room air, which is a Class 9 environment (see Table 12.3). The ante-room must have Class 8 or better air, the buffer room must have Class 7, and the direct compounding area (DCA), which is inside an engineered compounding workbench, must have Class 5.
Table 12.3 ISO Clean Air Classes by Airborne Particulate Amounts
Class |
≥ 0.5 µm/m3* |
Cleanroom Compounding Area |
|---|---|---|
ISO 1 |
0.0029 |
|
ISO 2 |
0.029 |
|
ISO 3 |
0.29 |
|
ISO 4 |
2.9 |
|
ISO 5 |
29 |
direct compounding area (DCA) |
ISO 6 |
290 |
|
ISO 7 |
2,900 |
buffer room |
ISO 8 |
29,000 |
anteroom |
ISO 9 |
290,000 |
room air |
* The maximum number of particles of different size ranges per cubic meter of compressed air. The cleanest air is the lowest class number while the dirtiest air is the highest. Cleanroom air for sterile compounding needs to be Class 5 or cleaner, while indoor room air is usually Class 9.
Anteroom
The anteroom is the preparatory room that comes before the buffer room. Prior to entering this room, the compounding technicians remove their outerwear and put on light scrubs. Once inside, they collect and prepare their ingredients and supplies, wash, and put on their compounding garb. As noted, the anteroom must be maintained at nothing less clean than an ISO Class 8 environment, so the air quality level needs to be periodically checked.

The anteroom serves as a supply preparation area and washing area, and as preparation for the buffer room.
Only necessary supplies, furniture, and equipment (such as a stainless steel table, supply transport carts, and the refrigeration and freezer units for the ingredients needing cold storage and completed CSPs) are allowed in the anteroom. It also holds pens, calculators, cleaning supplies, and nonrefrigerated CSP ingredients and packaged supplies. Many state standards require a “touch-free” sink and a “touch-free” non-wood door between the anteroom and the cleanroom.
The Buffer Room
The inner room to which the anteroom leads is known as the buffer room (it is technically the actual “cleanroom,” which is sometimes called the “IV room”). The buffer room is able to maintain the required cleaner air standard (ISO Class 7) than the anteroom because it is separated physically from the heavy foot traffic of the main hospital and pharmacy by the anteroom.
 Practice Tip
Practice Tip
Garbing and degarbing should never occur at the same time in the same place.
Only properly trained and garbed pharmacy personnel have access to the buffer room. The design elements include heat-sealed floors and smooth, nonporous walls and ceiling tiles that are easily cleaned. It contains only the items absolutely necessary for sterile compounding, including the stainless steel counters and workstations. The temperature and humidity of this room are maintained at specific levels to inhibit the potential for bacterial growth.
 Pharm Fact
Pharm Fact
The HEPA filter removes particles that are 0.3 microns in size, which is about 300 times smaller than the average diameter of human hair.
Within the buffer room are the primary engineering controls (PECs), which are the ventilated workbenches that have the DCAs within them. As cited, each DCA must have ISO Class 5 air quality. The ISO Class 5 environment is maintained within the PEC by its high-efficiency particulate airflow (HEPA) filter system that forces sterile, unidirectional air across the work surface of the DCA. The HEPA filters are 99% efficient in removing particles as small as 0.3 micrometers (microns) in size. The different kinds of sterile compounding PECs will be described later in the chapter.
 Safety Alert
Safety Alert
The technician should keep in mind that the greatest risk of contamination in a sterile compounding area comes from human error or touch contamination, despite the presence of equipment, filters, and engineering controls.

The SAS Air Sampler (which comes in different styles) is used to measure the air quality of the laminar flow workstation, buffer room, and anteroom.
Secondary Engineering Controls
The secondary engineering controls (SECs) for air quality are the ventilation and HEPA systems built into the cleanroom area’s heating, ventilation, and air conditioning (HVAC) systems. The SECs are calibrated to maintain the ISO Class 7 air quality in the buffer room as a whole and ISO Class 8 or better in the anteroom. Positive pressure is created when air is forced through the HEPA-filtered HVAC system into rooms of the sealed cleanroom. Positive pressure is required for a sterile compounding area to help keep contaminants from entering the cleanroom. The unidirectional airflow sweeps particles away from the room where CSPs are being compounded toward the lower-pressure anteroom and then outward toward the lowest-pressure outer area (see Figure 12.2).
As new air is HEPA-filtered and cycled into the room by the HVAC system, it replaces the old air in the room. The air replacement is called an air exchange. The rooms must have a USP-specified amount of air exchanges per hour. This is another way the air quality is maintained.
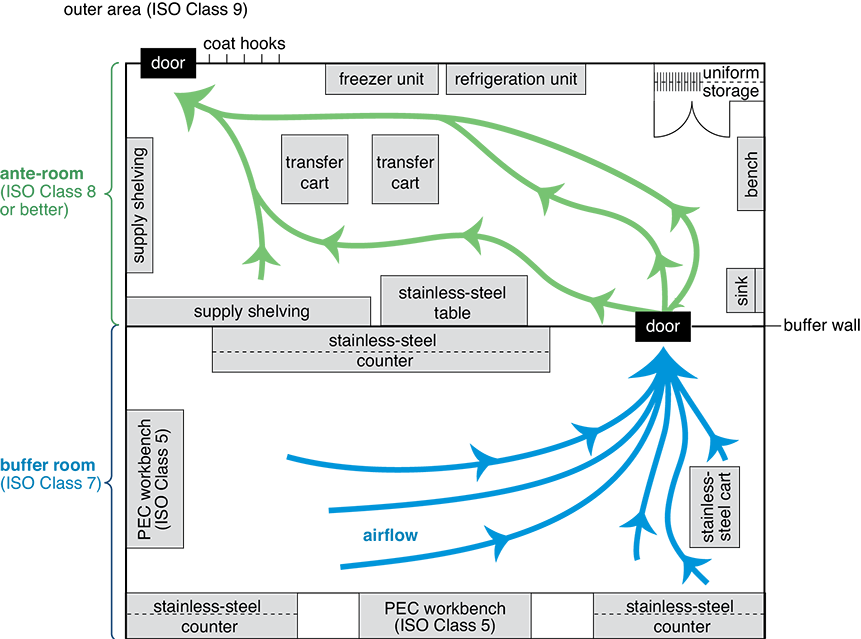
Figure 12.2 Sterile Cleanroom Facility Layout
Compounding technicians enter the anteroom (ISO Class 8) and, after preparation, enter the buffer room (ISO Class 7) to work in the DCAs (ISO Class 5) of the PECs. Notice that the airflow goes in the opposite direction, carrying air and any contaminants out of the high positive pressure buffer room through the lower pressure anteroom and to the outer area.
Cleanroom Facilities without a Separate anteroom and Buffer Room
Some hospital and inpatient care facilities may not have the space for a full anteroom and a buffer room with all the filtered ventilation systems for their sterile cleanroom compounding. Though it is not ideal, some establish a semi-enclosed segregated compounding area (SCA) away from the outer doors, windows, traffic, sink, and garbing areas. Some hospitals also are erecting small enclosed, ventilated, modular HEPA-filtered cleanroom compounding areas within larger rooms. Hospitals using SCAs and modular cleanrooms have to follow the USP <797> guidelines that fit those types of spaces.