12.7 Aseptic Technique
Despite the engineering controls, the sterile compounding area will not remain clean if sterile compounding personnel introduce contaminants into the environment from their skin, hair, jewelry, and clothing. One of the primary focuses of aseptic technique is to prevent carrying microorganisms and other contaminants into the work space and CSPs.
Aseptic Preparation
Before even coming to work in sterile compounding, a technician has to prepare. Sterile compounding personnel must not wear jewelry, cosmetics, perfume, artificial nails, or nail polish. These substances can flake and compromise the aseptic environment. No earbuds, headsets, devices, or cell phones are allowed in the cleanroom. If you have jewelry that cannot be removed, it must be covered.
 Practice Tip
Practice Tip
Prior to entering the anteroom, compounding technicians remove all cosmetics, jewelry, outer garb, and anything else they have with them. They can store these items in lockers and put on light scrubs.
Individuals who have certain medical conditions that increase the risk of contamination during the compounding process are also banned from the sterile compounding area. These conditions include communicable diseases, respiratory colds or infections, weeping sores, oozing tattoos, rashes, and sunburn. Food and beverages are not permitted in either the anteroom or the cleanroom, and you must not chew gum or engage in horseplay in these areas. Street clothes should be removed and clean scrubs donned right before entering the anteroom.
 IN THE REAL WORLD
IN THE REAL WORLD
In the fall of 2015, Duke Raleigh Hospital was undergoing renovations but it needed to keep the hospital open. So it chose to take advantage of the new mobile cleanroom created by Germfree Labs that was completely suited to USP Chapter <797> standards at the time. Duke Raleigh was the first US hospital to use a mobile unit. The unit is a combined hospital pharmacy cleanroom (with anteroom and buffer room with laminar flow workstations), and a hazardous compounding room (with Class II biological safety cabinets) in a specially designed semi-trailer facility. This unit allowed Duke Raleigh Hospital to have uninterrupted service during its renovations.
The possibilities, though, for this transportable cleanroom and hazardous compounding station go beyond hospital renovations. The mobile cleanroom offers great hope for hospitals and facilities in disaster-hit areas where the pharmacy has been damaged. It also offers hope for rural areas and even hospitals in developing countries that cannot afford a full cleanroom facility.

Many key duties have to be fulfilled in the anteroom before garbing, such as gathering supplies and finishing any calculations on dilutions. The technician places the pharmacist-approved label, compounding directions, and calculations along with the medication order (if needed) in a clear plastic bag and wipes it down with sterile 70% isopropyl alcohol (IPA) so that the bag can come into the cleanroom. The PEC cleaning supplies, compounding ingredients, and supplies are also wiped down and placed in a sanitized, nonporous molded plastic or stainless steel bin on a stainless steel cart for transport into the buffer room. Then the technician is ready to garb.
 Safety Alert
Safety Alert
The order for putting on sterile compounding personal protective equipment (PPE) is dependent on the placement of the sink and facility specific standard operating procedures (SOPs).
Aseptic Garbing and Washing
The garbing process for sterile compounding personnel follows a strict sequence. You put on the protective garb in a specific order: first shoe covers, hair cover, and face mask. Then comes handwashing and arm scrubbing. The sterile gowns (with sleeves) and gloves must be the cleanest items, so they are put on last; the gown goes on in the anteroom, and the gloves are donned in the buffer room.
Aseptic Handwashing and Hygiene
Aseptic handwashing is a more vigorous and thorough washing than the CDC handwashing guidelines. The aim of aseptic handwashing is not only to reduce the number of resident and transient flora to a minimum but also to inhibit their regrowth for as long as possible—not just on the hands, but also on the wrists and forearms. It means performing specific hand-washing procedures with unscented soap (plain or antimicrobial) and water followed by an alcohol-based sanitizer, as will be explained below.
 Safety Alert
Safety Alert
Sterile compounding personnel who prepare CSPs are required to perform aseptic handwashing, a more rigorous handwashing practice than the general guidelines dictated by the CDC.
Hand and Forearm Washing
The most ideal sink situations are those with a touchless faucet controlled by a foot pedal or an electronic sensor. Proper handwashing includes first removing any debris under the fingernails with a nail pick, sponge, and cleaner under warm running water. Then, using unscented soap and water, rub your hands together vigorously, cleaning each finger individually and the webbing between each finger for 30 seconds at a minimum.
 Pharm Fact
Pharm Fact
Skin cells are naturally shed at the rate of 30,000 to 40,000 every hour and a million cells per day, and with them come multitudes of microorganisms. Thousands of bacteria live on a square centimeter of skin, which is why washing, scrubbing, and garbing are so important!
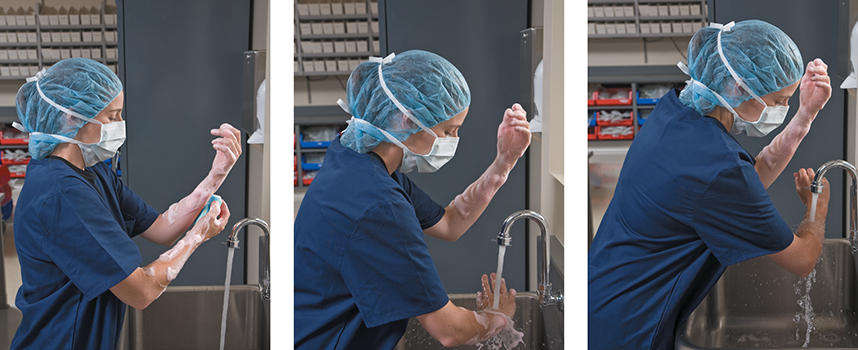
Clean the back of each hand by starting at the wrist, moving up the forearm to the elbow in a circular motion (see Figure 12.3). Rinsing should be done in the same order, working from the fingers down the forearms with the hands always pointed upward to allow the water to wash toward the elbows and off. The aseptic hand-washing technique radically affects how effective the process will be in terms of infection control, so it must be understood and done properly as practiced in a lab.

The pharmacy technician demonstrates putting on the face mask before handwashing, scrubbing the arms, and adding the gown.
The cleansing sequence should end with drying your hands on a lint-free disposable towel until they are completely dry. After donning a low lint gown, then apply an alcohol-based hand wipe, gel, or spray (where the liquid evaporates) for sanitizing hygiene. This entire hand-washing process must be completed multiple times during a shift in the sterile compounding area, including:
upon entering the sterile compounding area,
after a major contamination,
after touch contamination, such as scratching your face,
after eating, sneezing, coughing, or using the restroom, and
after every 30 minutes during batch CSP procedures.
Figure 12.3 Proper Aseptic Forearm Washing
This technician is completing the forearm scrubbing and rinsing sequence.
Sterile Gowning and Gloving
After the handwashing and hygiene, the last two procedures are gowning and gloving. With clean hands, open the sterile gown package (which has been wiped down), and put it on, one arm at a time. Be sure the gown does not touch the floor or any surfaces. Secure it in the back. Once gowned, sanitize your hands with sterile 70% IPA, and now you may safely enter the buffer room with the supply cart to be gloved.
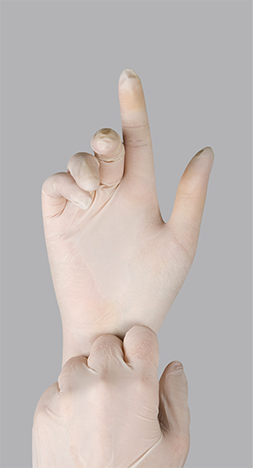
Gloving by the pharmacy technician provides an extra safeguard to touch contamination, but gloves too can become contaminated.
Gloving To finish the hand hygiene and disinfect the hands from using the cart and doors, the technician applies a long-lasting antimicrobial alcohol-based rub again and lets it dry before donning the sterile gloves. The gloves should be powder-free and compatible with alcohol disinfectants. The gloves are labeled “left” and “right.” The outer area of the gloves should never be touched. Grasping the inside of the cuff, the first glove should be pulled over the hand and the cuff of the gown so that no skin shows. Then the second glove should be put on in a similar way. Gloves must be worn at all times inside the cleanroom, especially while preparing CSPs.
The gloves need to be sanitized and inspected regularly during a shift for tears or punctures, and, if they are found, the gloves must be replaced with a new pair. In addition, every time a glove touches a nonsterile surface or object while you are working in the cleanroom or DCA, the glove must be sanitized with sterile 70% IPA spray. Gloves should also never be washed or reused. Discard them after leaving the cleanroom.
 Safety Alert
Safety Alert
Pharmacy personnel should not use petroleum-based ointments, creams, or lotions before donning sterile gloves, as these products may decrease the integrity of the sterile gloves.
Quality Assurance and Quality Control
Within the cleanroom facility, hospital quality assurance and quality control programs are implemented. Site inspections of the sterile compounding areas are required to initiate and maintain accreditation. Also required are quality monitoring and improvement procedures.
 Practice Tip
Practice Tip
If a technician leaves the sterile compounding area (to use the restroom, to go to lunch or breaks, and such), then the entire garbing and hand-washing process must be repeated.
Aseptic Technique Testing
Prior to working in the buffer room, each technician who is training in sterile compounding procedures must successfully pass a competency evaluation and a fingertip sampling three separate and distinct times. To do this, immediately after gloving, the technician places gloved fingers on a petri dish medium (agar) for growing cultures, and the dish is monitored to see if any microorganisms grow over the next seven days. If they do, that means that the glove was contaminated and the aseptic procedure was not done properly.
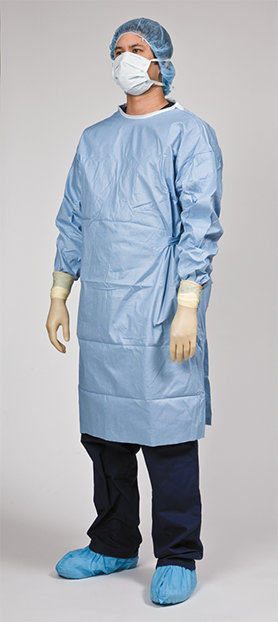
The full protective clothing of a technician helps keep CSPs sterile. After a shift, the aseptic garb must come off in the anteroom and be discarded properly in the reverse order of donning it.62
Compounded Sterile Preparation Testing
In addition to the fingertip testing of sterile compounding personnel, the quality control (QC) program in a sterile compounding area involves a sampling of the CSPs and drug products by the pharmacist before their release to the nurse and administration to the patient. Periodically, products will be sent for sterility testing—an established laboratory procedure for detecting viable microbial contamination in a sample or preparation. Release testing involves the final visual check of the CSP by the pharmacist for particulates, foreign matter, or discoloration against a lit background. If detected, the CSP is immediately discarded.
Ongoing Technician Testing
Sterile compounding technicians are observed by their pharmacist supervisors and given periodic aseptic testing. The testing frequency is dependent upon the risk level of the CSPs, facility SOPs, and USP <797> guidelines. A technician’s CSPs also receive periodic spot testings. The technique of all sterile compounding personnel must also be re-evaluated at a minimum of every six months. With such training, certifications, and experience, technicians are rewarded in higher salary for their higher-level, CSP-related responsibilities.
Degarbing
Upon completion of the CSP(s), the technician will return to the anteroom for the pharmacist check (if not done in the buffer room). If no one is in the process of garbing, the technician can properly discard the used garb. (If someone is preparing to enter the cleanroom, she or he must wait to degarb until the new compounder has entered the buffer room.)Unless otherwise instructed, the technician removes the PPE in the opposite order as putting it on: gloves, gown, face mask, hair cover, and shoe covers.