12.8 Sterile Compounding Supplies and Their Care
There are a variety of commonly used supplies that sterile compounding personnel work with. These include IV solutions, ampules and vials, syringes and needles, IV administration sets, medication cassettes, and personal pain pumps. Since ampules and vials have special ways to open and use them, they will be described in the next chapter on the processes of sterile compounding.
Solution Bottles and Bags
Sterile compounding facilities often use sterile water or manufactured mixtures of 5% dextrose in water, 0.9% normal saline, and other solutions as a medication base or diluent. Technicians choose the proper base in the correct bottle or IV bag based on the medical order, and, once in the DCA, they use the right-sized syringe and needle sets to inject the drugs, diluents, or nutritional substances into these bases. (Ways these solutions are used will be explained in more depth in Chapter 13.)
Syringes and Needles
To withdraw solutions from sterile containers or inject solutions into sterile containers, a needle-and-syringe unit is necessary. Technicians use this syringe transferal process to reconstitute a powdered medication, dilute an existing medication, withdraw fluid from a vial or ampule, or transport fluid from one container to another.
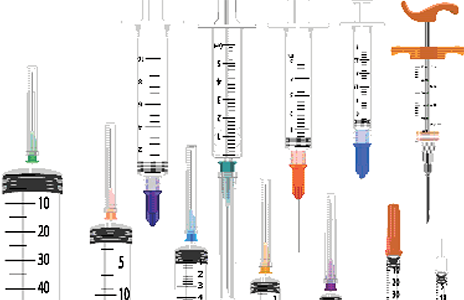
Various sizes of syringes used in sterile compounding range in volume from 60 mL (left) to 1 mL (right), with different incremental measuring units to calibrate them.
Syringes
Syringes are made of plastic or glass. Most sterile compounding syringes are made of sterile plastic components that are disposable. Glass syringes are expensive, and, since they are rarely reused, they are selected only when a drug is incompatible with plastic or when certain chemotherapy products are prepared. (In rare compounding scenarios, glass syringes may be cleaned and sterilized in an autoclave for reuse.)
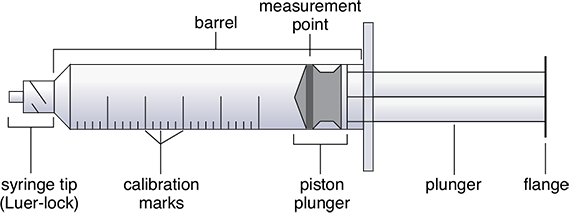
The main components of a syringe include the syringe tip, the barrel containing the calibration marks, and the rubber-tipped piston plunger (see Figure 12.4). Because the piston plunger, the inner plunger shaft, and the syringe tip must always remain sterile, they must never be touched, not even with a sterile glove.
Syringe Selection and Measurement Various sizes of syringes deliver significantly different volumes. During the sterile compounding process, choose the smallest syringe possible to hold the desired volume while being at least three-quarters full. A syringe is considered accurate to half of the smallest calibration mark (usually mL or cc) indicated on its barrel.
 Safety Alert
Safety Alert
Always be careful to touch only the barrel and top of a syringe and never the piston plunger, inner plunger shaft, or syringe tip, even with a gloved hand. If they are touched, they need to be thrown away.
To get an accurate measurement reading, you need to know the total volume and the number of marks between labeled measurement units. For example, the 10 labeled calibration marks on a 1 mL syringe indicate that each mark is equivalent to 1/10 of 1 mL (0.1, 0.2, 0.3, . . .). The small lines in between measure an equal proportion of that 1/10 unit. On a 50 mL syringe, each labeled calibration equals 10 mL, with each small line marking 1 mL. It is important to study syringes to learn their individualized calibration measurements.
 Practice Tip
Practice Tip
Always measure the amount of liquid in the syringe by aligning the shoulder of the plunger with the desired calibration mark on the syringe barrel.
To measure the volume of solution drawn into a syringe, air bubbles must first be tapped out. The volume is measured not where the rubber plunger tip lines up to a calibration line, but where the measurement edge of the rubber piston lines up to a calibration (see Figure 12.4).
Figure 12.4 Syringe Components
 Safety Alert
Safety Alert
Only Touch the shield of the needle. Never touch any of the metal—shaft, heel, bevel, or tip—or the needle is contaminated and must be thrown away in the sharps container. Besides, the needle is obviously sharp and could pierce your glove and skin.
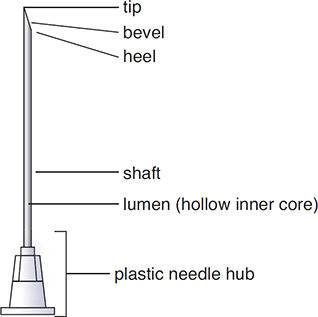
Needles
Sterile compounding needles are made of stainless steel or aluminum and are individually wrapped to maintain their sterility. A needle consists of several parts: the needle hub, lumen, shaft, heel, bevel, and tip (see Figure 12.5). A needle has a hard plastic cap that covers the shaft and tip until the needle is ready to be used. Even with gloves, this cap is the only place that can be touched. Accidentally touching any part of the needle itself leads to contamination and requires the disposal of the needle in a sharps container (secure needle disposal bin).
Figure 12.5 Needle Components
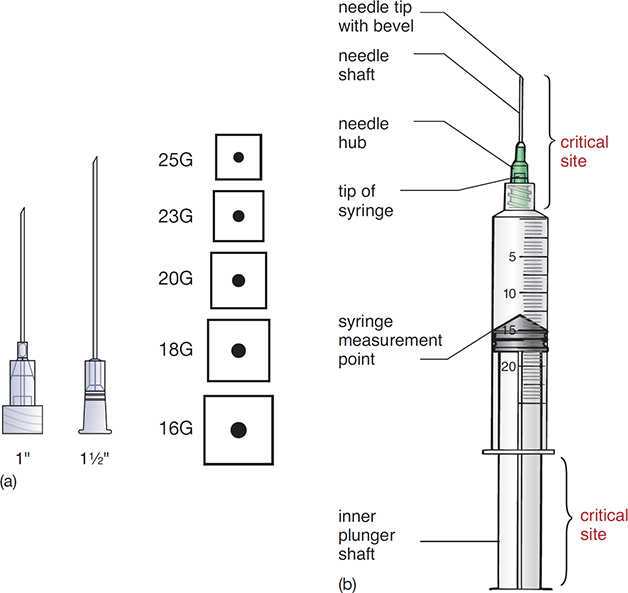
A needle’s size is determined by its length and gauge. The needle’s gauge refers to the diameter of the shaft’s hollow inner core and opening, or lumen. There is an inverse relationship between the gauge number and the size of the lumen. For example, a 16-gauge needle has a wider diameter than a 25-gauge needle. The smaller gauge numbers (with thicker lumens) are appropriate for thicker, more viscous liquids, and the higher gauges (thinner lumens) are used for injecting less dense solutions.
In sterile compounding, 1-inch- and 1.5-inch long needles are commonly used, and they have needle gauges ranging from 16 (wider) to 25 (narrower), as shown in Figure 12.6a.
The critical sites of a needle and/or syringe are the parts that include fluid pathway surfaces or openings (see Figure 12.6b). These sites must never be touched.
Figure 12.6 Common Needle Lengths and Gauges in Sterile Compounding and Critical Areas of a Needle and Syringe
(a) Needle lengths and gauges
(b) Critical sites
 Pharm Fact
Pharm Fact
IV sets do not have expiration dates, but they do contain the following legend: “Federal law restricts this device to sale by or on the order of a physician.”
Administration Sets and Their Components
Sterile compounding personnel sometimes use IV administration sets to prepare CSPs. An IV administration set is a sterile, disposable device of many linked components that is used to deliver the IV fluids. These fluids flow from the bag through the tubing and other components through an injection port with a needle into the patient’s vein. Often called “IV tubing” or an “IV set,” the assembled unit comes in a sterile, sealed package.
Sterile compounding personnel may use IV administration sets to:
transfer fluids between containers while working in a PEC
prime IV sets to prepare hazardous drug CSPs for patient administration
Regardless of the manufacturer, IV administration sets have certain basic components (see Figure 12.7), which will be described in more depth:
a universal spike adapter (commonly called a “tubing spike” or “spike”), which pierces the stopper port on the IV solution bag
a drip chamber, which allows healthcare personnel to view and count the drops of IV fluid before the solution flows down the tubing
flexible tubing, which delivers the fluid
a roller clamp, which adjusts the flow rate of the IV fluid from the source container into the receiving container or the patient
injection ports, to infuse fluids with primary IV
in-line filters, to remove contaminants from the tubing
a needle adapter set, which attaches a needle or a catheter to the patient and the IV tubing set
Sterile medicated solutions are often delivered to a patient via an IV administration set.

Sterile medicated solutions are often delivered to a patient via an IV administration set.
Universal Spike Adapter
The sharp, plastic bag spike adapter is used to pierce the IV bag’s tubing port to attach the drip chamber and tubing. Some IV sets have an air vent with a bacterial filter cover positioned below the spike. This vent allows air to enter the bottle or bag as fluid flows out of it.
Figure 12.7 Sterile IV Solution and Administration Set
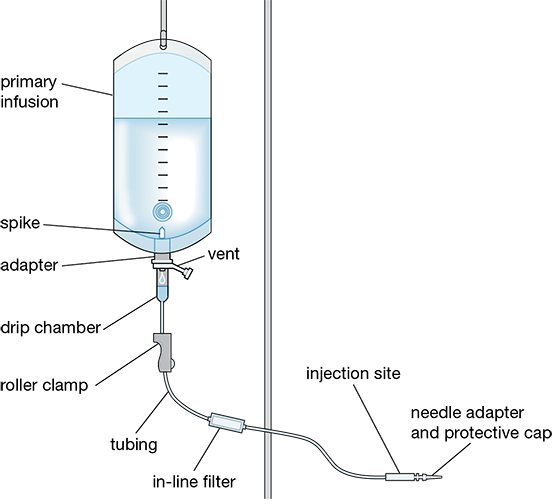
Drip Chamber
Sitting below the spike is the transparent, hollow drip chamber. The drip chamber allows any air bubbles to rise to the top of the IV fluid, thus preventing the air bubbles from entering the tubing and, subsequently, the patient. The drip chamber also allows the attending nurse to set the medication flow or infusion rate by counting the drops and then making adjustments to the pump, clamps, tubing, or height of the bag.
 Safety Alert
Safety Alert
If possible, do not use PVC IV sets for nitroglycerin, amiodarone, most biotechnology drugs, and fat emulsions due to the absorption of the drug by the plastic. PVC and DEHP-free IV bags and sets are also specifically recommended for pregnant women, neonates, children, ongoing IV patients, and other high-risk populations.
IV Tubing Types and Sizes
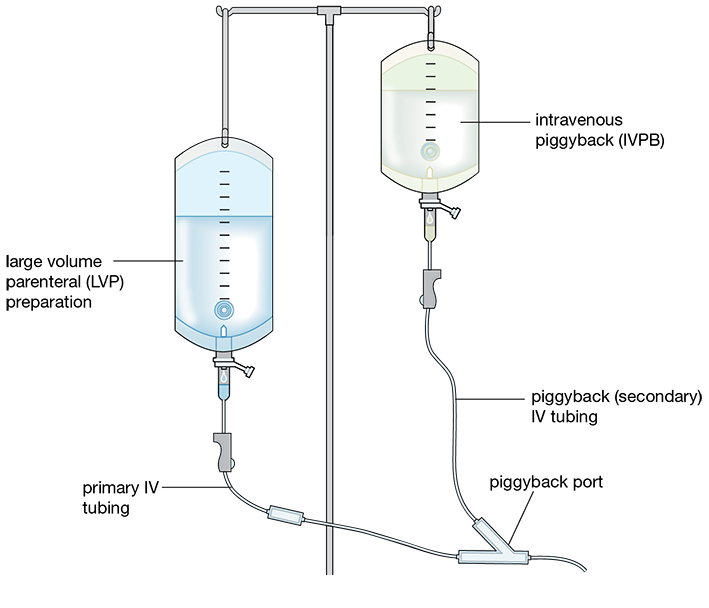
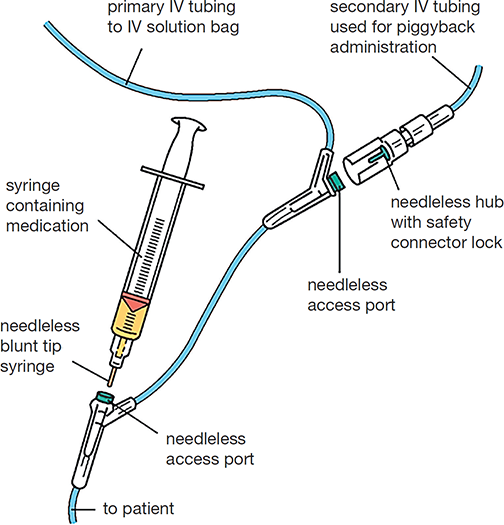
IV sets deliver either a primary IV solution or a secondary one. The secondary medication has a smaller bag with less volume. This medication is often more concentrated, and it needs to be diluted by the primary solution, so the secondary tubing is inserted into the primary tubing line to merge the liquids, which is called “piggybacking.” The nurse hangs the IV piggyback (IVPB)—another name for the secondary IV—higher than the first IV bag to allow the additional gravitational force to push the secondary IV solution into the primary line to the patient (see Figure 12.8).
IV Tubing Length The majority of the IV set is made of clear, flexible plastic tubing. The length of IV tubing in the sets varies, with extensions ranging from 6 to 120 inches. The length of tubing is dependent on the patient’s needs, the number of IV lines, and the procedure being performed. Longer tubing is used for primary IV lines in most hospital settings. Shorter tubing lengths are used for the IV piggyback secondary lines and in surgical settings where many people are working around a patient in a small space.
Figure 12.8 Primary Versus Secondary IV Lines
 Practice Tip
Practice Tip
Because so much of nitroglycerin is absorbed by PVC containers and IV sets, nitroglycerin is best compounded into a glass IV bottle and infused through a non-absorbing IV line. The same is true for vitamin A acetate, warfarin, methohexital, terbutaline, lorazepam, and insulin.
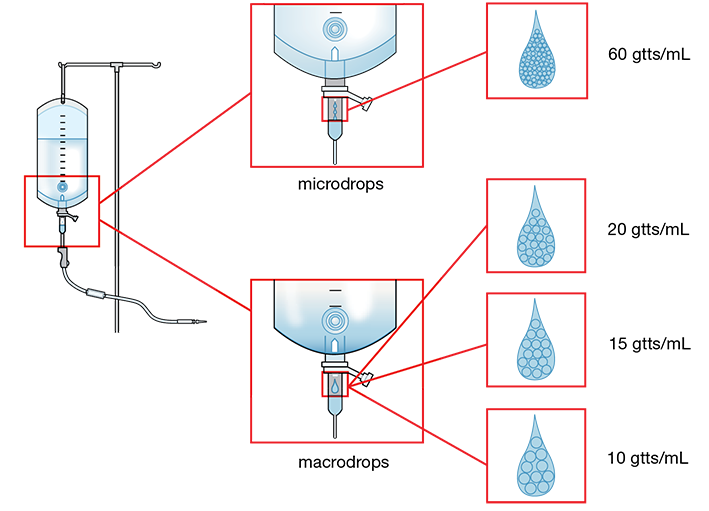
IV Tubing Diameter Size—Drop and Drip Factors The diameter size of the tubing selected is determined by the prescribed number of drops per mL the tubing needs to deliver. This calibration is referred to as the tubing’s drop factor, with larger diameter tubing delivering larger, fewer drops per mL, and smaller diameter tubing delivering smaller, more numerous drops (gtt) per mL. You will need to know this drop factor to determine how many bags will be needed for 24 hours or the next resupply. The equations to do these calculations will be addressed in Chapter 13.
The most common IV set sizes for adults are macrodrip tubing sets that deliver 10, 15, or 20 drops per milliliter—(10 gtts/mL, 15 gtts/mL, 20 gtts/mL as shown in Figure 12.9. IV tubing sets that provide smaller drops like 60 gtts/mL are used for pediatric patients and for particular IV medications requiring tight control. The smaller diameter tubing sets are called microdrip tubing sets (also known as minidrip sets).
Figure 12.9 Microdrip and Macrodrip Drop Sets
 IN THE REAL WORLD
IN THE REAL WORLD
When PVC tubing is manufactured or incinerated, dioxins, chloride, and other toxic compounds are produced and/or emitted, and a portion of these substances is usually released into air, water, or soil. Both chloride and dioxin are known toxins that affect organs and bodily systems and are carcinogenic. They can bioaccumulate in one’s body and the bodies of wildlife.
Because of these health and environmental issues, there is a medical move to go PVC-free. Europe is phasing out PVCs. Kaiser Permanente, one of the largest healthcare providers in the United States, and Dignity Health, the eighth largest healthcare system in the nation, are among those that have gone 100% PVC- and DEHP-free in their IV materials. Non-PVC IV tubing is available from most pharmaceutical manufacturers.
IV Tubing Plastic and Health Issues Some blood bags, IV solution bags, and IV tubing are made of a type of plastic known as PVC (polyvinyl chloride). Many common drugs, though, are partially absorbed by the PVC plastic tubing and/or IV bag. For example, when nitroglycerin is in a PVC IV bag and administration set, 70% of the drug is absorbed after 24 hours. This absorption reduces the amount of drug delivered intravenously to a patient.
Besides drug absorption issues, there are concerns about PVC chemicals leaching into the CSP solution and being absorbed by the patient. In addition, disposal of PVC causes environmental issues, and the chemical compound Di(2-ethylhexyl) phthlate (DEHP) often used to make the PVC tubing flexible creates other health issues. For these reasons, hospitals are moving toward non-PVC IV bags and tubing.
 Pharm Fact
Pharm Fact
B. Braun Medical and Hospira are just two of the manufacturers of non-PVC bags and tubing. Non-PVC alternatives are 30 times less toxic when disposed of by incineration and are significantly lighter, resulting in reductions in landfill costs.
Roller Clamp
The roller clamp contains a small roller that can move freely along the length of the tubing between the spike adapter and the needle adapter to manually regulate and adjust the solution flow rate. When the clamp is rolled up toward the universal spike adapter, the clamp loosens, increasing fluid flow and drip rate. When the clamp is rolled down toward the needle adapter, the clamp tightens, resulting in a reduced flow of fluid. The roller clamp is always closed when an IV solution is being attached to the tubing. There is also an auxiliary clamp, or slide clamp, that is used to completely stop the IV solution from flowing when needed.
In-line Filters
In-line filters are included in many IV administration sets. An in-line filter is a device used to remove contaminants flowing through the tubing, such as glass particles, fibers, and tiny bits of rubber that may have inadvertently entered the CSP during the sterile compounding procedures. The filters are built into the tubing or added to it and are classified by the size or nature of the particles they remove.
In-line filters vary in size and can minimize the risk of phlebitis or infection.
A 0.45 micron filter provides a final filtration of the fluid before it enters the patient, but it is not a sterilizing filter. Final filtration protects the patient against air bubbles, particulate matter, bacteria, and phlebitis. Phlebitis is vein inflammation caused by undissolved drug particles, plastic particulate matter from IV tubing, or infectious agents.
Manufactured IV solutions without drugs generally have very low particle counts and do not require filtration. However, when compounded drugs are added to these manufactured solutions, the particle count increases. A total parenteral nutrition (TPN) solution with multiple additives often requires an in-line filter.

This Y-site injection port allows a secondary IV or injection to be added to the main IV line.
Filters do not remove viruses as viruses are too small to be caught by the filter. However, a 0.22 micron filter is optimal for blocking bacteria. These filters, however, are so fine that they slow down infusion rates and can get clogged. IV solutions that contain a fat emulsion must utilize a larger micron size filter.
Injection Ports
A self-sealing injection port is needed to allow IV fluids or medications to be infused along with the primary IV into the patient’s vein through the tubing. The Y-site injection port (see Figure 12.10) provides a site for inserting the piggyback and IV push medications, reducing the pain of a new injection site for the patient.
Figure 12.10 Example of Y-site Injection Ports
These ports are used to administer various medications with the same primary IV set. A syringe can be injected into the port nearest the patient’s vein and a secondary tubing port can be connected to the line closer up the line to the drip chamber of the primary solution.
Catheter Set
A catheter set is located at the patient end of the IV set. The tubing delivers medication through the implanted needle in the patient’s vein. When an IV solution set is changed, the old tubing is removed from the catheter connection port and the new IV tubing is attached. This saves the pain and effort of repuncturing the patient each time.
Medication Reservoir Cassettes and Personal Pain Pumps
A new approach to sterile drug delivery is through devices that allow greater power to the patient in managing the rate of administration, especially in terms of pain medications. This delivery method is called patient-controlled analgesia (PCA)—or patient-controlled sedation (PCS). Personal infusion pumps can administer sterile intravenous admixtures that come stored in medication reservoir cassettes, such as those for the CADD-Prizm PCS II.
Experienced sterile compounding technicians will receive medication orders to prepare epidural and morphine cassettes. (Some drug manufacturers offer premixed sterile drug solutions in medication cassettes.)
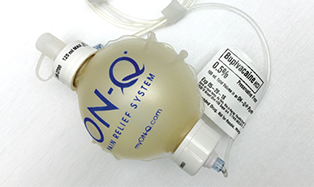

These CADD medication cassette reservoirs and the ON-Q* Fixed Flow Pump are examples of new carriers for sterile compounded medications.
Another new sterile delivery device is a small, solution-filled sterile plastic ball attached to a sterile catheter line that is sewn into a surgery site. The ball, such as the ON-Q* Fixed Flow Rate Pump, slowly deflates, administering the pain medication as the patient heals. Sterile compounding technicians fill these balls with the medications ordered for patients’ specific needs.