13.1 The Nature of Compounded Sterile Products
CSPs are often called “admixtures” because they are made by starting with a base solution that is compatible with human blood and adding medications and other ingredients to it. The body is primarily an aqueous or water-containing entity, so most CSPs and parenteral solutions consist of ingredients added to a sterile-water medium, a normal saline (NS) solution (0.9% sodium chloride with sterile water), or a base dextrose solution (sugar and sterile water mixture as in D5W). You, as a sterile compounding technician, will then add ingredients that include medications, electrolytes, vitamins, and nutrients. Electrolyte solutions contain micronutrients that carry important electrical energy for cellular function.
 Safety Alert
Safety Alert
It is essential that the base solution selected is compatible with the medication chosen. The measurements must be calculated correctly in order to maintain the capability between the diluent and the solute and between the CSP and the patient’s blood.
To assimilate properly into the patient’s blood supply, CSPs must be sterile while having chemical properties or characteristics that do not damage blood cells or vessels or alter the blood’s own chemical makeup. In other words, the CSPs must be compatible with blood in order to be safely administered. If they are not, they may cause a patient to have an adverse reaction. Sterile compounding technicians need a basic knowledge of these chemical properties to understand why the concentrations and measurements to compound a CSP must be followed so precisely—they have to be formulated to address these key necessities of the human body.
 Pharm Fact
Pharm Fact
Sodium chloride (0.9%) was originally called “indifferent fluid” because it had properties similar to human blood and did not cause visible cell rupture. It is now referred to as normal saline and is isotonic.
The chemical properties that must be matched to human blood include pH value, osmotic pressure, tonicity, and compatibility. Each of these concepts requires some explanation. As a sterile compounding technician, you will not be making these determinations, but each compounding pharmacist, manufacturer of IV ingredients, and Master Formulation Record considers these characteristics in the “recipes” for the parenteral solutions.
pH Value
The degree of acidity (acid) or alkalinity (base) of a solution is known as its pH value. Common household acids are vinegar and citrus juices. Bases include baking soda. All substances are on the spectrum from acid to base. Too intense an acid or a base can cause irritation or damage to the body, which is why it hurts if you get orange or lemon juice or baking soda in a cut.
Most high-school chemistry classes teach pH value by using litmus paper to test various solutions on a rating range of 0 to 14, with 0 as the most acidic. If the litmus paper turns pink when dipped, the solution is acidic—with a pH value less than 7.0. With a dark pink, lemon juice has a rating of 2.2; common white vinegar has 2.4; tomatoes, 4.5; and milk, 6.6.
 Put Down Roots
Put Down Roots
Osmosis comes from the Greek word õsmos, which means “impulsion” or “push.”
Blood plasma has a pH value of 7.4 and an osmolarity near 285 mOsm/L. Most IV solutions have similar osmolarity so as not to damage red blood cells.
If the litmus paper turns blue when dipped, then the solution is alkaline—with a pH value of more than 7.0. Baking soda (sodium bicarbonate) has a rating of 8.3; milk of magnesia has 10.5; ammonia, 11.0; and lye, 13, which turns litmus paper a very dark blue.
Blood plasma is near the very middle with a pH of 7.4; therefore, it is slightly alkaline. IV solutions with a pH near neutral (or near 7.0) are less likely to affect the natural pH of the blood.
Osmotic Pressure
Another key factor in parenteral solutions is managing osmosis. Osmosis is the natural flow of molecules in a solution through semi-permeable cellular or organ walls to equalize the concentrations of minerals on both sides. Proper osmotic pressure is needed to maintain a state of balanced movement across cellular membranes so as not to burst any membranes from undue pressure. Solutions cannot be too concentrated with solutes (dissolved ingredients) because the pressure to move those solutes through the cellular walls too fast will burst the walls.
 Pharm Fact
Pharm Fact
In laboratories, the osmotic pressure of solutions is measured on various kinds of osmometers. One type of osmotic measurement uses osmoles per liter (Osm/L) or milliosmoles per liter (mOsm/L).
Generally speaking, an IV solution must be iso-osmotic, meaning that the solution should have the same number of particles in solution per unit volume producing the same osmotic pressure as blood plasma, or blood fluid. IV solutions with too high a concentration of solute particles can cause blockages, so these solutions need to be diluted or administered to larger veins. These highly concentrated solutions can also be administered in a small volume parenteral (SVP) solution via an IV piggyback line that flows into a large volume parenteral (LVP) primary line of sterile normal saline or medication.
 Put Down Roots
Put Down Roots
The Greek word isos, means “equal to” or “the same as,” and iso-osmotic means equal to the osmotic pressure of blood plasma, which is considered the universal normal for all human solutions. Isotonic comes from the additional Greek word tonos, which means “tone” or “level.”
Complicated chemical equations are used to calculate different measurements of a solution’s osmotic characteristics. Osmolarity refers to the concentration of all molecules—those that cross the cellular membranes and those that do not—in a volume of fluid. Osmolarity is measured in milliosmoles per liter (mOsm/L). The osmolarity of blood plasma, for instance, is approximately 275–300 mOsm/L. The osmolarity of a solution affects whether water flows out of a cell or into a cell to equalize osmotic pressure, or remains equal in the osmotic exchanges of water and solutes.
Tonicity
Blood has to have a certain level of water content to be healthy, as do organs and individual cells. Low water levels indicate problems, such as dehydration or high glucose levels associated with uncontrolled diabetes.
Tonicity is the directional flow pattern of water in cells based on concentrations and osmotic pressure within their environments. The tonicity of a solution determines whether it catalyzes an inward flow, an outward flow, or a balanced molecule exchange between the inside and outside of a cellular wall. A solution’s tonicity is classified as isotonic, hypotonic, or hypertonic (see Figure 13.1 and Table 13.1).
 Name Exchange
Name Exchange
Maintenance solutions typically refer to fluids used for maintenance therapy. Maintenance therapy replaces the ongoing losses of water and electrolytes under normal physiologic conditions via urine, sweat, respiration, and stool.
Isotonic Solution
An isotonic solution has a similar concentration of solute particles to blood plasma, prompting an exchange of water molecules through the semipermeable cell walls with equal flow moving inward and out. There is no net loss or gain of water or electrolytes in the cells. An isotonic solution that has a similar pH to blood will flow even more easily through the system without damage. Examples of isotonic solutions are NS (0.9% sodium chloride) and lactated Ringer’s solution (a parenteral nutritional solution). At times, a pharmacist may need to check and adjust the tonicity of parenteral preparations to ensure that they are isotonic or nearly isotonic.
Figure 13.1 Tonic Conditions in Cells
When a cell is bathed in an isotonic solution, the amount of water entering and leaving is the same. In hypotonic solutions, more water enters a cell and may result in a cell rupture. In hypertonic solutions, more water leaves a cell and may result in cell shriveling and dehydration.
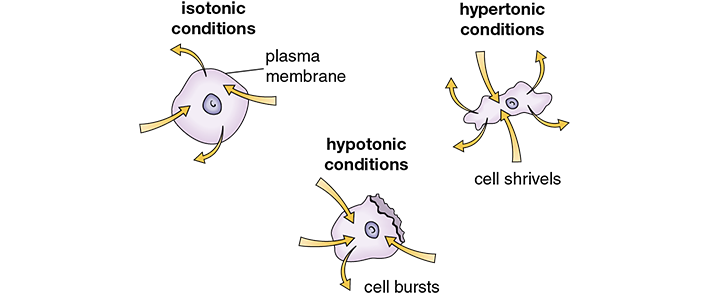
Table 13.1 Common Parenteral Solutions and Tonicity
Tonicity |
Solution |
Osmolarity per Liter |
|---|---|---|
Isotonic solutions |
Dextrose 2.5% in 0.45% NaCl (D2.5W in ½ NS) |
280 mOsm/1000 mL |
Dextrose 5% in sterile water (D5W) |
300 mOsm/1000 mL |
|
0.9% NaCl (normal saline [NS]) |
310 mOsm/1000 mL |
|
Lactated Ringer’s solution (LR) |
275 mOsm/1000 mL |
|
Hypertonic solutions |
Dextrose 5% in 0.45% NaCl (D5W in ½ NS) |
405 mOsm/1000 mL |
Dextrose 5% in 0.9% NaCl (D5W in NS) |
560 mOsm/1000 mL |
|
Dextrose 10% in sterile water (D10W) |
600 mOsm/1000 mL |
|
3%, 5%, 14.6%, and 23.4% NaCl concentrates |
Varies |
|
Hypotonic (hydrating) solutions |
0.45% NaCl (½ NS) |
155 mOsm/1000 mL |
Dextrose 2.5% in sterile water (D2.5W) |
140 mOsm/1000 mL |
Hypotonic Solution
A hypotonic solution has fewer solute particles than blood plasma and cells so water is drawn into the cells to equalize the differing concentration, causing the cells to swell. The directional flow of molecules is into the cell. This is important for individuals who are dehydrated, such as those with diabetic ketoacidosis or those who are low on minerals. The flow should be inward to replenish the depleted cells. An example of a hypotonic solution is 0.45% sodium chloride (sometimes called NS).
 Safety Alert
Safety Alert
Patient recipients of hypotonic and hypertonic solutions must be closely monitored for potential risks, as these types of solutions are short-term therapies to quickly rebalance a system. Hypotonic solutions can cause a potentially life-threatening electrolyte imbalance. Hypertonic solutions can cause collapse of cells and veins.
Hypertonic Solution A hypertonic solution has more solute particles than the blood plasma and cells themselves, so water is drawn out of the cells, which causes them to shrivel. This can be helpful to reduce swelling in cells or to draw toxic elements out of the cells. On the rare occasions when hypertonic solutions must be administered to patients, these infusions must be done cautiously and gradually by an experienced nurse or a physician, or the infusions can cause congestive heart failure. Examples of hypertonic solutions are TPN solutions, 20%–50% dextrose in water (D20W to D50W), and 3%–5% sodium chloride (3% saline to 5% saline). Hypertonic solutions often require a slower infusion rate or administration via a larger vein to prevent phlebitis (inflammation of the veins).
Chemical Compatibility
Finally, each compounded sterile IV solution must have chemical compatibility with the blood, with other medicated solutions in the body, and among its own ingredients. Chemical compatibility is the ability of two or more base components (or additives within a solution) to combine without resulting in significant physical or chemical reactions to the components or the encountered solutions. For example, fat emulsions are incompatible with electrolyte solutions. Trissel’s Stability of Compounded Formulations is an excellent reference for checking IV drug incompatibilities. The book also provides stability and storage information in monographs on many compounded drug formulations.

 Practice Tip
Practice Tip
Product package inserts also provide information on the properties of each medication and directions on how to mix and use it to create and maintain compatibilities.