13.5 Completing Compounded Sterile Preparation for Storage and Delivery
After the various types of CSPs are prepared and labeled, but before they are transferred for short-term storage or immediate delivery, they must be inspected once more by the technician and the pharmacist and assigned a BUD on the label (if not preprinted), with a sign-off from both the preparer and the pharmacist. As noted, the USP Chapter <797> guidelines specify procedures for dating, labeling, storage, handling, packaging, and transporting of CSPs in the hospital or infusion pharmacy. Improper procedures could adversely affect the sterility or stability of the CSP.

Medications compounded by the technician must await approval by the pharmacist along with all the syringes, vials, and ampules used.
The Final Physical Inspection and Verification
The technician checks to make sure there are no precipitates, incompatibilities, or leaks in any of the IVs, then lays out all the CSP and all the components used in the compounding for the pharmacist’s approval. The pharmacist’s final CSP inspection will include checking for accuracy in the proper selection and quantities of ingredients, technique for aseptic mixing and sterilization, packaging, and labeling or reviewing photos that have been taken with dedicated cameras in the clean room that have been used to capture the volumes and medication drawn up and added to the final container.
Physical appearance of the CSP is a key element for inspection. As explained, often more than one drug must be added to an LVP IV solution. Once those medications are reconstituted with sterile water (or normal saline) and then mixed with the others in the base solution, there is always the possibility of a physical incompatibility or particulate contamination. If one medication is an acidic salt such as potassium chloride and the other an alkaline salt such as sodium bicarbonate, a solid precipitate such as sodium chloride (salt) may form when the two are mixed together in the LVP. To avoid any solid precipitates, all final CSPs are inspected by the pharmacist and the technician against a white or black background to confirm that precipitates (and particulate matter) are not present. If there is concern about a potential physical drug incompatibility, Trissel’s Stability of Compounded Formulations should be consulted.
If any particulate matter is identified, then the CSP is discarded to prevent the introduction of any particles in the solution into a vein that could cause an obstruction in a vein or a blood vessel. The use of SVPs that contain medication minimizes the risk of physical incompatibility, since the medications are not in prolonged contact with the additives or drugs in the LVP base solution.
Storage of Compounded Sterile Preparations
Once approved and labeled, the CSPs are either delivered or placed in short-term storage until delivery. The storage conditions vary according to the preprinted label instructions. The pharmacy technician is often responsible for monitoring and documenting the conditions under which CSPs are stored—at room temperature, or in the refrigerator or freezer. Each controlled temperature area must be checked daily and recorded in a temperature log. If a CSP has been exposed to temperatures that exceed the acceptable range (such as greater than 4 hours at 40 degrees), it must be discarded.
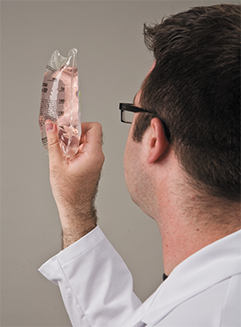
If a CSP has been stored before delivery, the pharmacist must re-inspect it for its physical properties to see that it has maintained its integrity.
Inspecting, Packaging and Handling
If not immediately delivered, the CSP must be re-inspected by the pharmacist before delivery for any defects, such as precipitation, cloudiness, or leakage, that may have developed during storage. The closure of the container must also be inspected for integrity. With any noted defect, the CSP must be discarded.
 Practice Tip
Practice Tip
General rule of thumb for all CSPs in terms of sterility or integrity is, “When in doubt, throw it out!”
How you package the CSPs for delivery can determine how well they maintain their physical integrity, sterility, and stability from this point on. The transport packaging must protect CSPs from damage, leakage, contamination, degradation, and particle adhesion while simultaneously protecting transport personnel from harm if hazardous compounds are present. Tamper-evident closures and seals on CSP ports can provide an additional measure of security. If the CSP is sensitive to light, light-resistant materials must be used. In some cases, the CSP should be packaged in a special container (e.g., a cooler) to protect it from temperature fluctuations.
At times, you must also select the best transport mode to deliver the properly packed CSPs to arrive in an unshaken, undamaged, sterile, and stable condition. The exterior packaging must be clearly labeled with the specific patient information and delivery handling instructions, along with the storage and administration counsel and any necessary highlighted warnings.
Priming and Administration of the IVs
After the CSPs are compounded and approved, a technician often delivers them to the nursing unit. Just prior to administration, the nurse will check that the CSP is still sealed and has its packaging and labeling intact. Then the nurse will hang the IV bags one at a time, keeping all ports and needles sterile. Priming is needed for both the primary and secondary tubing. Sometimes the technician does the priming, depending on the CSP, especially if it is a chemotherapy drug.
Bedside Priming
Priming is the action of letting fluid run through the system before administration, tapping at all the connections to dislodge bubbles to be sure that all of the air is out of both lines prior to attaching to the patient. The amount of fluid needed to prime the tubing depends on its length—up to 18 mL for longer primary sets, down to 3 mL for the shorter secondary extensions. Sometimes technicians do this priming just before delivery. See the presentation on priming the IV line at https://PharmPractice7e.ParadigmEducation.com/PrimingIV.
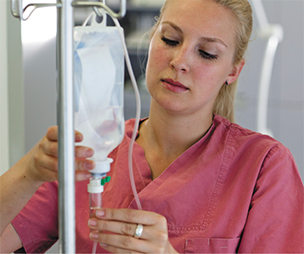
Before administering the IV, the nurse will prime the tubing (if not preprimed) to clear the lines of air bubbles and to check and calibrate the drip rate.