13.6 Premade IV Solutions Not Requiring Sterile Compounding
For some parenteral administrations, premade IV solutions are available. Recent innovative IV technologies from commercial pharmaceutical manufacturers, such as vial-and-bag products and frozen antibiotics, offer many advantages over cleanroom CSPs. They minimize measurement and compounding errors.
Vial-and-Bag Systems
A vial-and-bag system provides a single vial of powdered medication attached with an adapter to a specified IV bag solution that acts as the diluent. After scanning the product’s bar code, the nurse uses aseptic technique to break the seal of the attached powder vial, releasing the medication into the IVPB bag. This action “activates” the medication, which, once fully dissolved, is administered in short order to the patient via the secondary line tubing.
A vial-and-bag IV system is assembled by the technician but activated by the nurse at the patient’s bedside.
Though the technician does not compound the vial-and-bag product, it must be assembled (linking the vial and the bag with the connector device. Docking and activation of proprietary bag and vial systems in accordance with the manufacturer’s labeling for immediate administration to an individual patient is not considered compounding and may be performed outside of an International Organization for Standardization (ISO) 5 environment. Docking of the proprietary bag and vial systems for future activation and administration is considered compounding and must be performed in accordance with this chapter, with the exception of 14. Establishing Beyond-Use Dates. Beyond-use dates (BUDs) for proprietary bag and vial systems must not be longer than those specified in the manufacturer’s labeling.
Vial-and-bag systems are marketed under many trade names, such as ADD-Vantage (Pfizer) and Mini-Bag Plus (Baxter Healthcare) and addEASE (B. Braun Medical). Each vial-and-bag system differs somewhat in design, but the concept is similar. The vial of drug—which has not been reconstituted—is coupled (with or without an adapter) with an appropriate volume of IV solution. Minibags are usually available in 50 mL, 100 mL, and 250 mL sizes with many diluent options (D5W, D5NS, NS, lactated Ringer’s, and so on). However, only select products are available in vial-and-bag systems—mostly in-demand antibiotics.
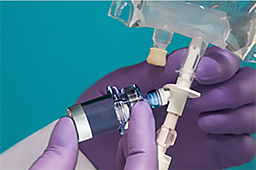
The technician assembles the vial-and-bag system in a cleanroom DCA, preparing the IV for the nurse to activate at the patient’s bedside when it is time to administer it. With different systems, there are various ways to insert the vial contents into the bag.
CSPs are a time-consuming and labor-intensive process. Though initially more costly, vial-and-bag systems offer a variety of benefits for healthcare personnel. In addition to fewer returns to the pharmacy and less wastage, other advantages are improved safety, efficiency, and cost-effectiveness.
Safety
Preparation errors are minimized with vial-and-bag systems because doses are standardized. Enhanced labeling and bar coding of both the vial medication and the IV solution are provided so the nurse knows exactly what is in the IV solution. The closed-system sterile packaging also minimizes the risk of contamination. Finally, these devices do not use needles, thus preventing the possibility of inadvertent needle sticks by the pharmacist, technician, or nurse.
Efficiency
Vial-and-bag systems are efficient because doses are premeasured for rapid reconstitution and easy assembly by the nurse. There is no need for freezing, thawing, or refrigeration of the solution in the pharmacy or the nursing unit because the product is immediately administered to the patient.
Cost-Effectiveness
With the vial-and-bag system, there are no additional personnel or supply costs incurred—such as for syringes, needles, gauze, and so on. With fewer plastic syringes and other supplies used, less waste is produced for disposal. There is also less wastage when an order is changed. The expiration dates of these nonactivated manufacturer products far exceed the beyond-use dating of a compounded product required by USP Chapter <797>, so they last longer. These advantages may well outweigh the additional cost per unit.
Frozen IV Solutions
Manufactured frozen IV solutions are premixed products and, consequently, are also not considered CSPs. Most frozen IV products are antibiotic solutions for SVPs. Like the vial-and-bag IV solutions, the frozen IV solutions are also more costly than compounding standard IV solutions and additives. However, hospital pharmacies purchase these frozen products for a number of the same reasons that they purchase the vial-and-bag systems—the longer expiration dates, reduced risk of microbial contamination, and less labor-intensive preparation for pharmacy personnel. The reconstituted vials of antibiotics in CSP solutions have much shorter half-lives than the frozen solutions, and thus there is potentially more wastage in CSPs. These benefits partially offset the higher acquisition cost and the additional freezer space needed for the frozen IVs.
Until a medication order is received, premixed IV products are kept in the hospital pharmacy freezer. When a frozen IV is ordered, it is thawed at room temperature or in the refrigerator (if there is sufficient time). The use of a warming bath or a microwave to expedite the thawing process (“forced thaw”) is not recommended. Manufacturers’ recommendations for thawing vary among products, so personnel must be sure to check each product’s label or package insert.
After thawing, the technician prepares a patient-specific label that includes the manufacturer’s expiration date. Again, the expiration date varies with the drug and storage conditions. For example, a frozen Ancef antibiotic solution has an expiration date of 48 hours at room temperature or 30 days if stored in the refrigerator. A Zosyn antibiotic solution has an expiration date of 24 hours at room temperature or 14 days if stored in the refrigerator. Once thawed, these frozen preparations cannot be refrozen.
After preparing the label, affix it to the bag for pharmacist review, and, once approved, send the medication to the nursing unit for administration.