2.4 National Oversight Agencies and Organizations
The laws, acts, and amendments previously discussed in this chapter provide the minimum level of acceptable standards for a broad scope of issues, offering a basic structure for the safe use of drug products and for the practice of pharmacy. Federal and state government agencies, along with nongovernmental organizations, work to ensure that the regulations required to fulfill these laws are established, reviewed, updated as needed, and enforced.
 Safety Alert
Safety Alert
The FDA regulates OTC labeling, so that it is understandable to a layperson.
Food and Drug Administration
The FDA’s Office of Medical Products and Tobacco oversees drugs. Within this office are several agencies pertinent to the practice of pharmacy: the Center for Drug Evaluation and Research (CDER), the Office of Pediatric Therapeutics, the Center for Devices and Radiological Health, the Center for Biologics Evaluation and Research (vaccinations), and the Office of Orphan Products Development. The profession of pharmacy is most affected by the CDER, which is primarily involved in the following tasks:
new drug development and review
generic drug review
OTC drug review
post-drug approval activities
As a public safety watchdog, the FDA enforces its regulations, including those for truth in advertising for food and drug packaging, labeling, advertising, and marketing. The FDA has been known to ask a manufacturer to cancel advertising campaigns and even to instruct the manufacturer to present a new advertising campaign to clear up any misconceptions. The ads and labels of OTC medications undergo the same level of FDA scrutiny to make all of the information readable and understandable to laypersons.
The FDA is also responsible for the annual publishing of Approved Drug Products with Therapeutic Equivalence Evaluations, better known as the Orange Book (available online). It identifies all drugs that are approved for both their safety and efficacy. This reference is used by drug wholesalers and in pharmacies primarily to make sure that generic products can be safely substituted for brand-name products.
The FDA offers two online publications, the Purple Book and the Green Book. The Purple Book (Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangability Evaluations) is a guide to approved bioengineered drugs. The Green Book (FDA Approved Animal Drug Products) is a guide to drugs for use in veterinary medicine. There will be more discussion on these references in Chapter 4.

Throughout its history, the FDA has striven to keep the public safe—through legislation, laboratory oversight, public education presentations, and undercover drug inspections. Here are two undercover FDA agents in the mid 1950s, William Hill and Charles Eisenberg, ready to intercept illegally trucked and sold amphetamines and barbiturates.
US Pharmacopeial Convention
The FDA works with the US Pharmacopeial Convention (USP)—an independent, nonprofit, scientific organization—and uses its quality standards for prescription drugs, OTC drugs, and dietary supplements. Their annual book of standards, or compendium, is called the US Pharmacopeia–National Formulary (USP–NF). All new drugs approved by the FDA must meet applicable USP–NF standards. The USP also has important works on dietary supplements, homeopathic products, and sterile and hazardous compounding standards.

The US Pharmacopeia-National Formulary is an important reference for pharmacists and pharmacy technicians.
Drug Enforcement Administration
The Drug Enforcement Administration (DEA) is the primary agency responsible for enforcing the laws regarding potentially addictive controlled substances, both illegal and legal. Every pharmacy, healthcare institution, warehouse, or business involved in any way with controlled substances must be registered with the DEA so that it can track the ingredients and control the drugs. One of the DEA’s functions is to inspect all medical facilities, including pharmacies, where suspicious activity is detected. The DEA works with state drug and narcotic agencies responsible for routine unannounced physical inspections; in addition, the local agency investigates issues of unsafe prescribing, dispensing, or forging of controlled drug prescriptions. The DEA can track the flow of narcotics from manufacturer to warehouse to pharmacy to patient.
The DEA—with the help of each state board of pharmacy and its agents—also keeps a close watch on prescribing and dispensing trends. For instance, any sudden increase in scheduled drug usage by a particular pharmacy (or in prescriptions by a particular physician) may trigger an investigation. Therefore, pharmacy personnel, including technicians, must be diligent in their handling and record keeping of controlled substances, as the misuse of these drugs has become a growing problem. Patient profiles must be carefully reviewed to monitor any overzealous use of controlled substances.

The Drug Enforcement Administration investigates suspect increases in drug usage.
Through audits, the DEA can determine if a pharmacy warehouse, for instance, is dispensing controlled substances outside the scope of its licensed registration. In a much publicized case in 2012, the DEA suspended the license of a Florida wholesaler for two years, claiming that the company neglected its primary responsibility to prevent the diversion of controlled substance medications from legal prescriptions to illegal distribution at two chain drug stores. These two pharmacies dispensed over forty times the usual amount of an extended-release form of oxycodone (OxyContin), a strongly addictive Schedule II opioid drug. Drug wholesalers generally set monthly limits on the quantities of scheduled drugs they will send to individual pharmacies. It is not uncommon for a wholesaler to refuse to sell and send high volumes of medications, such as alprazolam (Xanax) or tramadol (Ultram), to a community pharmacy at the end of the calendar month if they are near their limit. In such cases, if the drug is needed, the pharmacist or technician must identify an alternate source.
National Council for Prescription Drug Programs
The National Council for Prescription Drug Programs (NCPDP) is a not-for-profit organization of members in the pharmacy service industry that issues six-digit NCPDP Processor ID Numbers (BINs), which pharmacies and pharmaceutical providers use for online electronic records transmissions. Similarly, prescribers and pharmacies each have a National Provider Identifier (NPI), a unique 10-digit number, issued by the Centers for Medicare & Medicaid Services for government reimbursement. Pharmacies use these numbers in their FDA and DEA interactions and in ordering, purchasing, and tracking their medications, and in processing third-party prescription claims to insurance. The NCPDP has issued unique identifying numbers to more than 70,000 pharmacies. In addition to these numbers, it has established industry standards to facilitate online e-prescribing and exchanges of health information between pharmacies and medical offices. Also, pharmacists are eligible to request an NCPDP number, which may be needed for future pharmacy clinical service reimbursements.

This seal of approval appears on accredited online pharmacy websites indicating that they meet nationally approved standards of pharmacy practice and are complying with the state standards for their location.
National Association of Boards of Pharmacy
The National Association of Boards of Pharmacy (NABP) is the only professional organization that represents all 50 state boards of pharmacy. NABP has developed a model for state practice acts and regulations to help individual state boards of pharmacy build their own regulations. Their Model State Pharmacy Act and Model Rules of the National Association of Boards of Pharmacy streamlines the best of the differing pharmacy laws enacted in individual states to provide model language to the state boards of pharmacy. NABP has no legal regulatory authority but offers resources to assist state pharmacy boards as they put into place their laws, requirements, oversight, and enforcement.
NABP also operates the Verified Internet Pharmacy Practice Sites (VIPPS), an accreditation program for internet pharmacies recognized by 17 states. With thousands of rogue internet drug outlets—known as fake online pharmacies—distributing counterfeit and unapproved drugs, VIPPS was developed in 1999 to help patients in the United States find legitimate internet pharmacies. The VIPPS program addresses such issues as protection of the patient’s privacy, authentication and security of prescription orders, adherence to a recognized assurance policy, and provision of meaningful consultation between patients and pharmacists. NABP also performs an on-site survey of each VIPPS applicant’s pharmacy facility. NABP then posts a list of VIPPS online pharmacies, which can be found at https://PharmPractice7e.ParadigmEducation.com/NABP.
Only legitimate internet pharmacies and pharmacy-related websites qualify for “.pharmacy” domains. When patients find a site ending in “.pharmacy,” they will know that it is a safe and legal online pharmacy that has been vetted through NABP’s “.pharmacy” standards. “.Pharmacy” sites operating in a non-US country will also be vetted against that country’s laws, regulations, and pharmacy practice standards. More information and a list of approved entities with registered “.pharmacy” domain names, is available at https://PharmPractice7e.ParadigmEducation.com/SafePharmacy.
State Boards of Pharmacy
Governor-appointed leaders from the pharmacy community and consumer representatives make up the membership of each state board of pharmacy. The selected pharmacists generally represent community, hospital, and other areas of pharmacy practice. These boards work with the state departments of health and advise state legislators on pharmacy laws and regulations. Each state board is also responsible for overseeing the inspection of all new pharmacies and maintains a database of all active pharmacist licenses (and those of pharmacy technicians if required). Most states include the annual registration of pharmacy technicians in their database. They set the legal pharmacist-to-pharmacy technician ratio as well as oversee the licenses and certifications.
 Practice Tip
Practice Tip
Remember that laws and regulations vary from state to state. When a conflict occurs between a state and a federal law or regulation, the more stringent law or regulation always applies. Get to know your state laws.
 IN THE REAL WORLD
IN THE REAL WORLD
Pharmacy corporations or community pharmacies may elect to enforce more stringent control policies than those mandated by the state. For example, some pharmacies in locations experiencing high rates of drug abuse or OTC drug recreational use may implement a store policy requiring a written prescription from a licensed provider for the selling and dispensing of any OTC Schedule V drug.
Some state boards specifically authorize the scope of technician practice, whereas other states define what the technician may do by detailing what the pharmacist must do. In these states, by default, duties not legally mandated to the pharmacist may be carried out by the technician. For instance, many states allow any pharmacy technician to compound intravenous (IV) solutions under a pharmacist’s supervision, whereas other states require pharmacy technicians to be specially certified. Still other states restrict IV solution preparation only to pharmacists.
Each state has its own drug enforcement agency that works with the regional DEA and may have different priorities. As noted, the state boards of pharmacy often implement their own regulations that are more rigorous than the federal standards, especially in terms of controlled substances. For example, most states allow some mechanism for filling emergency narcotic pain-relieving medication for terminally ill hospice patients, but some do not.
For noncontrolled drugs, there are variances in state regulations as well. Typically a foreign physician (e.g., from Canada or Mexico) may not prescribe drugs to be filled in US pharmacies. However, some boards of pharmacy in states adjacent to the Canadian or Mexican border may allow dispensing if certain conditions are met. Even the dispensing of insulin syringes may be limited by state boards—some states require a prescription for the syringes in an effort to discourage illicit drug use.
Occupational Safety and Health Administration
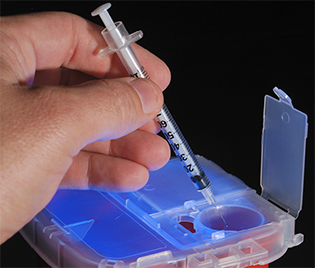
Technicians must follow OSHA rules to safely dispose of syringes to prevent the transmission of communicable diseases such as hepatitis and HIV. OSHA has many rules for hazardous compounding to protect staff from toxic exposure.
The Occupational Safety and Health Administration (OSHA) is an agency of the Department of Labor. Its primary mission is to ensure the safety and health of US workers. It establishes and enforces workplace regulations and standards; provides training, outreach, and education; and encourages continual improvement in workplace safety and health.
Pharmacy facilities must follow OSHA legal standards and regulations for their facility and processes, with all employees complying. OSHA even offers a video of a hospital pharmacy practice area and various elements to consider for safety at https://PharmPractice7e.ParadigmEducation.com/OSHAvideo.
Centers for Disease Control and Prevention
This federal agency, under the Department of Health and Human Services, provides guidelines to protect the public from large-scale health issues and illnesses. The Centers for Disease Control and Prevention (CDC) grew out of an army effort to fight malaria a century ago, which is still part of its work. The CDC works to fight infectious diseases to stop epidemics (regional widespread contagious diseases) and pandemics (globally widespread epidemics), sexually transmitted diseases, food-borne diseases, and other contagious illnesses. In addition, it focuses on educational awareness about health, disease, and addiction prevention, as well as environmental health, occupational safety and health, and injury prevention.
 Put Down Roots
Put Down Roots
Epidemic grew from the Greek word epi, “among,” and demos, “people.” Pan, in Greek, means “all,” giving us the word pandemic, a disease that affects all people, or an international epidemic.
Many vaccinations and prescriptions have been developed for these preventive health goals. Pharmacy technicians encounter CDC guidance in such things as hand hygiene practices and temperatures for vaccine refrigeration. Hospitals, because so many sick patients gather there, have to be particularly concerned about contagious diseases. This will be covered in the hospital practice unit in Chapter 12. The CDC makes recommendations, not regulations.
State Departments and Boards of Health
Each state also has its own department or board of health to oversee the public health of state citizens. These entities can establish their own regulations for infectious diseases and other health issues that can indirectly affect pharmacy practice. For instance, they can choose to quarantine certain individuals or set up vaccine clinics under emergency situations. They may enlist the help of pharmacies in working against state epidemics or public health threats. For instance, in 2014, Governor Andrew Cuomo convened a gathering of state agencies and major hospitals to have an emergency response plan for the Ebola health alert. In this, pharmacists and technicians would have the job of preparing increased medications within the hospitals but not direct patient contact.

Theodor Geisel, before he became the beloved Dr. Seuss, used his talents to make posters and films for army troops. This poster was made for the Malaria Control in War Areas program, which evolved into the CDC.