13.2 Overview of the Sterile Compounding Process and Components
As noted, there are many steps in the sterile compounding process, many of which are complicated. The process begins with the medication order, which generates a drug utilization review and a label with all its components. The process ends with verification by the pharmacist and delivery for nurse administration. It is helpful to get a sense of the full sweep of the steps and components before exploring each more closely. Table 13.2 provides a summary.
Table 13.2 Overview of Steps in Sterile Compounding
This is a general outline of process steps for sterile compounding in a two-room cleanroom area. Each facility is slightly different, based on its layout and standard operating procedures. It is essential to take a sterile compounding course with practice labs to get a real sense of the detailed steps involved. USP Chapter <797> is the ultimate source for all sterile compounding guidelines. |
|
The Medication Orders and Sterile Compounding Considerations
Medication orders for CSPs are delivered to the hospital pharmacy from the physician via handheld devices, computers, faxes, pneumatic tubes (like in banks), or hand delivery (see samples in Figure 13.2). Once the hospital pharmacy receives the CSP order, a pharmacist (or in certain facilities, a senior technician) enters the order into the pharmacy’s computer system. However, many hospitals utilize computerized physician order entry (CPOE), and the prescriber may have already entered the order into the computer system. Either way, the entered order prompts software to run a Drug Utilization Review (DUR). The DUR will check the order against the patient’s medical history and medication profile to alert the technician of any allergies, cross-sensitivities, drug-drug or drug-food interactions, duplicate therapies, or contraindications.

Sterile compounding requires special training and equipment.
The pharmacist resolves all computer-generated warnings and either modifies the order with the physician or approves it, and the sterile compounding labels are generated. Rather than just issuing a single label for the medication order, a separate label is issued for each individualized dose to be compounded. These are provided to the sterile compounding technician in the pharmacy.
Figure 13.2 Common Physician’s Orders for Parenteral Solutions
These are medication orders with sterile compounding sigs and abbreviations. To translate, see Table 13.3.
 cefoxitin 1 g IV every 6 h over 24 hours
cefoxitin 1 g IV every 6 h over 24 hours
 nafcillin 1 g IV every 4 h
nafcillin 1 g IV every 4 h
 penicillin 2 million units IV every 4 h
penicillin 2 million units IV every 4 h
 add 100 units Humulin R regular insulin to 500 mL NS @ 20 mL/hour (label ℞ concentration 0.2 units/mL)
add 100 units Humulin R regular insulin to 500 mL NS @ 20 mL/hour (label ℞ concentration 0.2 units/mL)
 begin magnesium sulfate 5 g in 500 mL NS to run over 5 hours × 1 dose only
begin magnesium sulfate 5 g in 500 mL NS to run over 5 hours × 1 dose only
 change IV fluids to 0.45 NS with 20 mEq KCl @ 125 mL/hour
change IV fluids to 0.45 NS with 20 mEq KCl @ 125 mL/hour
In addition to the patient-specific medication orders, the sterile compounding technician is also generally responsible for preparing all of the ongoing CSPs needed for a day. This is called the daily IV run, or the batch—a 24-hour supply of every CSP for each patient in a unit, a wing, or the hospital. (A “batch” can also mean the preparation of specified amounts of CSPs for storage and future use.) Labels are generated for each dosage in the batch.
Since parenteral solutions are the core products ordered in sterile compounding, you must become familiar with the different kinds, the common ways of compounding them, and the abbreviations for terms used for parenteral solutions (see Table 13.3).
Three basic forms of parenteral IV solutions may be ordered; the large-volume parenteral, small volume parenteral, and parenteral nutrition solutions. The large and small volume parenterals are the most commonly ordered. There are key distinguishing features that affect how each parenteral is compounded and administered.
Large-Volume Parenteral (LVP) Solutions
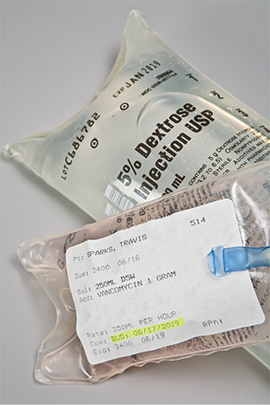
These solutions, which are administered through primary IV bags and tubing sets, replenish fluids and provide drugs, electrolytes, and nutrients to patients. Large-volume parenteral (LVP) solutions are generally administered over a prolonged period that ranges from 8 to 24 hours, so they are available in 250 mL, 500 mL, and 1,000 mL IV bag sizes. Since electrolytes carry important electrical energy for cellular function, an LVP usually contains one or more electrolytes that have been added to the IV solution. Potassium chloride is the most common electrolyte, but other salts of potassium, as well as magnesium or calcium, can be added based on the requirements of the individual patient. Other common additives to LVPs include trace elements, vitamins, and minerals. (See Figure 13.3 for a sample of an LVP label.)
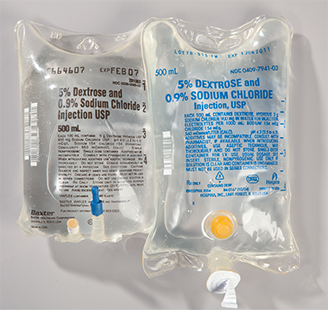
Dextrose 5% in normal saline (0.9%) is a common 500 mL large-volume parenteral IV (as is the 1 L [1,000 mL size]).
Figure 13.3 Large-Volume Parenteral Solution Label
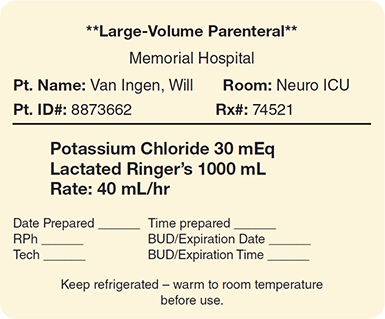
Table 13.3 Commonly Used IV Products and Abbreviations
Component |
Abbreviation |
|
|---|---|---|
Fluids |
2.5% dextrose in water |
D2.5W |
5% dextrose in water |
D5W |
|
5% dextrose and lactated Ringer’s solution |
D5RL or D5LR |
|
10% dextrose in water |
D10W |
|
5% dextrose and 0.9% sodium chloride |
D5NS |
|
2.5% dextrose and 0.45% sodium chloride |
D2.5 ½NS |
|
5% dextrose and 0.45% sodium chloride |
D5 ½NS |
|
0.9% sodium chloride; normal saline |
NS |
|
0.45% sodium chloride |
½NS |
|
Lactated Ringer’s solution |
RL or LR |
|
Sterile water for injection |
SW for injection or SWFI |
|
Bacteriostatic water for injection |
BW for injection or BWFI |
|
Sterile water for irrigation |
SW for irrigation |
|
Normal saline for irrigation |
NS for irrigation |
|
Electrolytes |
Potassium chloride |
KCl |
Potassium phosphate |
K phos or KPO4 |
|
Potassium acetate |
K acet |
|
Sodium phosphate |
Na phos or NaPO4 |
|
Sodium chloride |
NaCl |
|
Additives |
Multivitamin for injection |
MVI |
Trace elements (combinations of essential trace elements such as chromium, manganese, and copper) |
TE |
|
Zinc (a trace element) |
Zn |
|
Selenium (a trace element) |
Se |
 Practice Tip
Practice Tip
Depending upon the patient, medications may also be added to an LVP, such as insulin or famotidine (Pepcid).
One common LVP solution that contains a specific mixture of electrolytes—called lactated Ringer’s solution (LR)—is used often for fluid restoration after blood loss from injury, surgery, or burns. LR is a mixture that includes sodium chloride, sodium lactate, potassium chloride, and calcium chloride. It may be used alone or in combination with a dextrose solution. Other types of medications that may be administered by LVP include nutritional solutions and many chemotherapy drugs, both of which will be explained in more depth later in the chapter.
Small-Volume Parenteral (SVP) Solutions
Because this IV solution has less volume and contains a concentrated prescribed medication, it is either administered directly to the patient by being syringed into the port of the primary IV line or “piggybacked” into the IV tubing of an LVP using a secondary IV line (as described and diagrammed in Chapter 12).
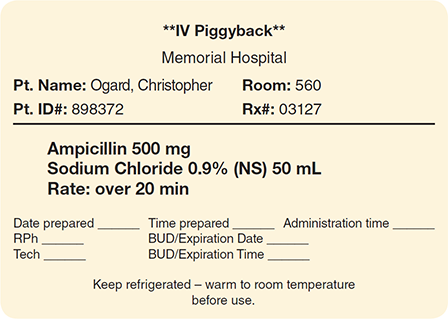
The majority of small-volume parenteral (SVP) solutions prepared by sterile compounding personnel are IV piggybacks (IVPBs). They are comprised of medication injected into a minibag (such as 25, 50, 100, or 150 mL) of a base solution such as sterile NS or D5W. The solution volume needed is dependent upon the drug’s dose and solubility, according to the manufacturer’s recommendations or the Master Formulation Record. (See Figure 13.4 for a sample SVP label.)
An SVP is typically administered intermittently over a short period ranging from 10 minutes to an hour (sometimes longer), based on the physician’s or the manufacturer’s recommendations. Since the IVPB line is connected to a port in the primary IV line, this secondary line can be turned on and off for the required periods of intermittent drug administrations. To obtain a more gradual release over an extended time, some medications may be mixed in the LVP to obtain a more dilute concentration, thus avoiding possible phlebitis and patient discomfort.
Parenteral Nutrition Solutions
These IV solutions are specialty LVPs. Parenteral nutrition (PN) is a means of supplying needed nourishment to patients who cannot or will not take in sufficient (or any) food or water through their mouths or a port in the GI system. Parenteral nutrition can be peripheral parenteral nutrition (PPN) or total parenteral nutrition (TPN). PPN provides partial nutritional support for shorter-term hospital stays; TPN provides complete and sustaining nutritional support for patients who are unconscious or who cannot receive food, water, or medication by mouth (designated on the medical order as npo). This inability to take nutrition by the mouth can be the result of surgery, infection, or inflammation in the GI tract. TPN administration is generally for extended stays in the hospital (or sometimes even at home with home health care).
Figure 13.4 Small-Volume Parenteral Solution Label
Sterile Compounding Label Components
The medication label provides patient identification information, such as the patient’s name and ID number (or bar code), and the patient’s hospital room or unit number. In addition, the sterile compounding labels must also contain very specific information about the product, dose, dosing interval or infusion rate, storage requirements, beyond-use date (or expiration date if premanufactured), and initials of preparers.
Often the label includes directions. The label and formatting will differ among hospital pharmacies and software vendors, but labels generally include information contained in Table 13.4.
Table 13.4 Sterile Compounded Labeling Requirements
Each compounded product must have affixed upon it a label that encompasses most or all of the following information: |
|
Note: Consult the hospital pharmacy’s policies and procedures manual for an institution’s specific labeling requirements. |
Solution, Drug, and Additive Information
Each CSP requires a base solution and additives, and the label must mention both. For the base solutions, you need the name, concentration, and amount. For instance, the base solution listed as 0.9% NS 1 L (1,000 mL) indicates the concentration (0.9%, or 900 mg of saline per 100 mL of fluid), the type of solution (NS), and the amount (1L or 1,000 mL).
The label must also list each additive and the amount of each. A compounded product may have more than one additive. For example, a TPN solution may have as many as fifteen or more additives, including electrolytes, minerals, vitamins, and trace elements (and occasionally antacids).
Administration Directions for Infusion Rates, Intervals, and Times
The medication administration time or infusion rate (the rate in mL/hour that it takes for the IV bag to be fully infused in the patient) may be indicated by the medication order written by the physician, or it may be determined by consulting the manufacturer’s package insert. If the medication’s administration is not ordered by the physician and the package insert is unavailable, the pharmacist may find this information by consulting one of the pharmacy reference manuals, such as the Handbook on Injectable Drugs (available in print or digital online format).
For CSPs, the most common medication timing information for LVPs is noted as the infusion rate (see Figure 13.3 earlier). The physician usually orders an infusion rate for an LVP in milliliters per hour, such as 125 mL per hour. At this rate, one liter (1,000 mL) of the LVP will run for approximately eight hours.
Occasionally, the physician will order an IV infusion rate based on the amount of drug per hour. For instance, the infusion rate of heparin is often ordered in units per hour, such as 1,000 units per hour. Once the administration time or infusion rate has been calculated, the pharmacy is then responsible for ensuring this information is added to the label. (The calculations of infusion rates come later in this chapter.)
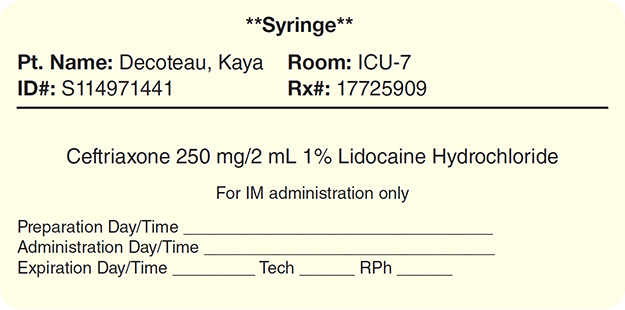
In general, only IV piggyback (IVPB) and IV push (drug injection directly into a vein via syringe) medications require a specific administration time as in Figures 13.4 and 13.5. For instance, cefazolin 1 g IVPB may be administered at a specific time using an IV pump. A phenytoin sodium (Dilantin) 500 mg IV push is administered by a nurse or a physician who slowly injects over a 10-minute period the solution into the patient’s IV line at specified 6-hour intervals. The administration time varies widely between different types of medications and the route of administration or dosage of the drug.
Figure 13.5 Syringe Label
The label may also indicate that the entire solution needs to be administered (to address potential overfill issues, which will be described later in this chapter).
Storage Requirements
Storage requirements (such as refrigerate, keep frozen—thaw immediately prior to administration, do not refrigerate, or protect from light) must also be included on the label, per the medication order, Master Formulation Record, or manufacturer’s instructions. The storage conditions of the CSP will often dictate the beyond-use dating, along with USP Chapter <797> guidelines. Typically, sterile compounds stored in a refrigerator or freezer have a longer beyond-use date than those stored at room temperature. Many IV medications must be refrigerated (or even frozen) to maintain their ability to provide the full effects for therapy. Once a CSP is thawed, it cannot be refrozen. Other IV solutions must be covered with an amber-colored bag to protect the drug from exposure to light. An example of a light-sensitive agent is an IV infusion of the antifungal drug amphotericin B.
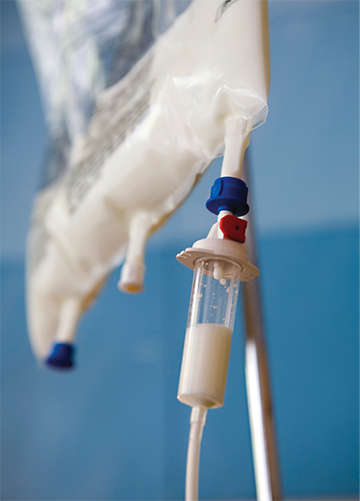
A parenteral nutrition solution containing fatty acids (Liposyn) gives the IV a white color. The solution should be refrigerated for storage.
Beyond-Use Dating
For each manufactured IV solution, the label bears an expiration date, which is based on the product’s stability under the designated storage conditions. However, once a technician in a sterile compounding facility adds a medication such as penicillin to a manufactured stock IV bag, that IV bag is now a CSP, and it requires a calculated beyond-use date (BUD) based on USP <797> guidelines, which is far shorter than the manufacturer’s expiration date. The BUD is the date and time after which the CSP may not be used as determined from the day and time the sterile compound was prepared and how it was stored. The drug’s stability, or its ability to maintain integrity in its compounded form, is affected by the number of ingredients as well as the storage conditions.
Usually the label software automatically generates the BUD, but, when using the Master Formulation Record, the BUD will need to be determined by the technician and checked by the pharmacist. For most CSPs, the beyond-use dating is counted in hours rather than in days and months. The possibility of microbes multiplying within a CSP increases over time as the physical and chemical stability and integrity of the product decrease. Preservatives, or ingredients to extend integrity, are not in single dose vials. The determination of BUDs is complicated, and one must refer to the USP Chapter <797> for how it was prepare and how it was stored. The guidelines consider the environment where the compound was made, the starting ingredients, and the storage conditions of the CSP. It will be important to always read and follow the most current USP Chapter <797> guidelines.
Auxiliary Labels
In addition to the compounding label, the pharmacist can recommend that auxiliary labels be added to call the nursing staff’s attention to unique conditions of the CSP, such as an unusual administration route (like an epidural or via a central catheter); proper storage conditions; special IV tubing; a change in administration procedure or dosage concentration; or a coupling with commercially available products. Special high-alert medications such as heparin and cancer drugs require such stickers to minimize any chance of a medication administration error. Table 13.5 presents some examples of the many auxiliary labels available.
Remember, expiration dates are used for manufactured products while BUDs are used for CSPs, according to USP guidelines.
Accountability on Label
Every CSP label must also identify the compounding personnel with their initials in the provided blanks to allow traceability and accountability.

The IV technician is comparing the CSP label to the medication order to verify accuracy.
The blank headings vary widely among institutions. In some facilities, the label may provide separate blanks, one preceded by prep by, preparer, or tech, and the other blank preceded by checked by or RPh. Some labels simply have blank spaces where both the technician and pharmacist must sign their names.
Master Formulation Record
Some pharmacy labeling programs provide sterile compounding instructions directly on the label. For instance, the label might contain instructions that direct the IV technician to draw up 5 mL of a 1.36 mEq/mL concentration of calcium chloride 10% solution in order to provide a 6.8 mEq dose of the medication. (See Chapter 6 on calculating milliequivalents.)
However, per the guidelines in USP Chapter <797>, a Master Formulation Record (or control record) must be followed when the labels do not provide compounding instructions and also when CSPs are prepared in a batch for multiple patients. This information is contained in the pharmacy software. As in nonsterile compounding, the Master Formulation Record is like a tested and approved recipe that lists the ingredients, specific procedures, equipment to be used, and testing required for each specific kind of CSP (see Table 13.6).
Table 13.5 Auxiliary Labels Commonly Used in Sterile Compounding
Auxiliary Labels with Blanks for Required Information |
|
|
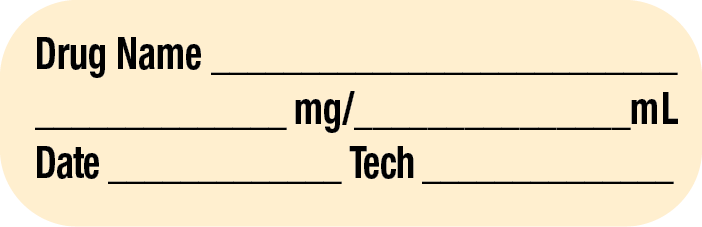
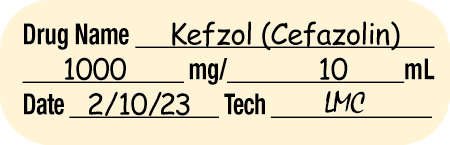
These labels are for powdered drug vials: one label is blank and one is completed by the sterile compounding technician, who attaches the label to the powdered drug vial upon dilution; in addition to recording the drug name, the final drug concentration, and the date and time of dilution, the technician diluting the powdered drug records their initials. |
|
|
Some facilities use two auxiliary labels to provide information similar to that provided on the larger labels above. |
Preprinted Auxiliary Labels |
|
|
This label indicates that a CSP should be delivered to the nursing unit and administered to the patient immediately. |
|
This label indicates that the medication is to be used as needed. |
|
Storage labels highlight how the CSP must be treated to maintain integrity, potency, and stability. |
|
This warning label reminds nurses that they do not need to give additional insulin to the patient because it is included in the CSP delivered. |
|
This label warns that special cautionary procedures should be applied because of a dangerously high concentration (most often used on bulk potassium chloride bottles). |
|
This label alerts handlers that this CSP contains potassium chloride, a potentially dangerous drug. |
|
This label cautions that a CSP is to be administered only for irrigation of a wound, dressing, tube, etc. |
Note: This table presents but a small sample of some of the most frequently used CSP auxiliary labels. Many other auxiliary labels are used in sterile compounding. |
|
Table 13.6 Sterile Master Formulation Record Components
A Master Formulation Record must include at least the following information: |
|
The Compounding Record
For sterile products that are assembled according to the manufacturer’s direction, such as standard reconstitution of powders for injectable medications, no Compounding Record is required. However, for every compounded product using a Master Formulation Record, a Compounding Record, or log, must be established by the technician on the computer for each patient, as in nonsterile compounding. This record documents the ingredients, calculations, and compounding process, allowing for traceability. It is critical that the record also describes in detail any deviations from the label directions or the Master Formulation Record and any compounding problems or errors experienced. Each Compounding Record must be reviewed and approved with the pharmacist’s signature or initials and the date before the CSP is released. See Table 13.7 for recommended components of the sterile Compounding Record.
Table 13.7 Sterile Compounding Record Components
Compounding Records must include at least the following information: |
|
If applicable, the Compounding Record must also include: |
|