13.8 Hazardous Sterile Compounding
A subcategory of sterile compounding is hazardous compounding. Cancer-fighting, or antineoplastic, drug therapies generally apply toxic agents to kill or inhibit the cancer cells, which are faster-growing than normal healthy cells. However, these are not the only hazardous agents. Destructive compounds also include antivirals, hormones, dangerous biological agents, and radiation treatments (nuclear pharmacy). Any drug that is known to have a risk of at least one of the following six criteria is considered a hazardous drug (HD):
carcinogenicity (causes cancer)
teratogenicity (causes development damage/problems)
reproductive toxicity (causes reproductive damage/problems)
organ toxicity (causes organ damage/problems)
genotoxicity (causes genetic damage)
new drugs that mimic hazardous drugs in structure or toxicity

Many cancer treatment drugs are very toxic. Patients often lose their hair, but it will grow back after treatments are completed.
Many pharmacy technicians, especially those working in a hospital setting, occasionally handle hazardous agents, especially in compounding nonsterile and sterile medications. Others specialize in hazardous or nuclear compounding.
 Put Down Roots
Put Down Roots
The term carcinoma, meaning “cancerous tumor,” originated from the Greek words carcinos, meaning “cancer,” and onkos, meaning “mass.”
Clearly, there is no acceptable level of exposure to HDs. Recent scientific studies have shown that even those handling the toxic agents in receiving and delivery of ingredients and final products experience far more exposure than was previously known. The CDC has a special center for hazardous substances: the National Institute for Occupational Safety and Health (NIOSH). The CDC and OSHA have published the NIOSH Alert: Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings, and the ASHP has established its own new HD guidelines. The USP Chapter <800>, which was finalized in 2016, had to be fully implemented by institutions doing hazardous compounding before December 1, 2019.
USP <800> outlines requirements for receipt, storage, mixing, preparing, compounding, dispensing, and administration of hazardous drugs to protect the patient, healthcare personnel, and environment. This chapter can offer only an overview. For more information about USP <800>, go to https://PharmPractice7e.ParadigmEducation.com/USP800.
Figure 13.11 Safety Data Sheet
Safety Data Sheets (SDSs) on each toxic agent with colorful pictographs about the danger level and response must be available to employees, according to OSHA rules, USP <800> (2016), and the Globally Harmonized System (GHS) of classification and labeling of chemicals.

According to USP <800>, personnel in a hospital or compounding facility who come into any level of contact with HDs require specialized equipment, training, protective equipment, and procedures for the handling, preparation, and disposal of these substances. Hospitals are required to develop written policies and procedures for each aspect of HD use to be in compliance with the USP and the Joint Commission, as well as state and federal regulations.
Safety Data Sheets (SDSs) for each hazardous agent or investigational drug must be readily accessible so that employees can see what they are handling and what should be done in the case of an accidental exposure or spill (see Figure 13.11). For an extended explanation, see Chapter 10.
Risks of Exposure
NIOSH categorizes risk for exposure to hazardous agents through four routes: (1) trauma, (2) inhalation, (3) ingestion, and (4) direct skin contact. The routes are illustrated by the following examples:
Trauma or injury: A technician uses a syringe to add a drug to an IV bag and accidentally gets pricked with the needle or receives a cut from a broken container of the hazardous drug.
Inhalation of the hazardous substance: A technician drops and breaks a bottle containing a volatile substance or uses poor manipulation technique with a vial, thereby releasing a fine mist and inadvertently inhaling the toxic substance.
Ingestion: A technician gets minute powder particles on lips when crushing an oral tablet or even cleaning a counting tray and unconsciously licks lips.
Direct skin contact: A technician accidentally spills a medication when pouring it from a large container into a smaller container or flask, and a drop splashes their skin. Direct contact with many cancer drugs can cause immediate reactions, such as the following:
Doxorubicin can cause tissue death and sloughing if introduced into a skin abrasion.
Nitrogen mustards can cause damage to the eyes, mucous membranes, and skin.
Within the hospital pharmacy, many activities have potential to result in HD exposures through the various routes without care and protection (as seen in Table 13.9).
Table 13.9 Routes of Exposure to Hazardous Agents
|
The use of unprotected, incorrect technique in HD compounding not only poses contamination risks for the patient, but also jeopardizes the technician’s own health and the area and people around them due to cross contamination.
With exposure to HDs, workers can suffer acute, chronic, and long-term health consequences if proper precautions, procedures, and training do not take place. The acute responses include skin rashes or allergic reactions. In women and men of reproductive age, chronic exposure can result in infertility, miscarriages, or neonates with low birth weight or congenital malformations. Long-term chronic exposure for all workers includes a higher risk for certain cancers, including skin and bladder cancers and leukemia.
Medical Surveillance
To prevent the serious health issues that can be caused by exposure to HDs, a medical surveillance program by the hospital or the compounding facility should be implemented according to USP <800> guidelines. The goal of medical surveillance is to collect and interpret data on HD workers to detect any changes in their health status due to potential exposure. The surveillance is meant to identify the earliest reversible biologic effects in every worker so that exposure can be identified, eliminated, or reduced before the employee sustains irreversible damage.
The program consists of an initial baseline of health (including reproductive history); exposure assessment; monitoring of health through regular physical examinations, laboratory tests, and questionnaires; response plans when there is evidence of HD toxicity; and an exit physical exam on termination of employment. If exposure-related health changes occur, it should prompt immediate re-evaluation of primary preventive measures (e.g., administrative and engineering controls, PPE, and others). In this manner, medical surveillance acts as a check on the effectiveness of controls already in use.
Any person of reproductive age who routinely works with hazardous agents must confirm in writing an understanding of the the reproductive risks of handling hazardous drugs. A pharmacy technician who is pregnant, breast-feeding, or trying to conceive should notify their supervisor so that so that they can discuss if they want to be reassigned to a different department position, take extra precautions, or have different work responsibilities, so as to minimize contact with any hazardous substances.
Personnel Training
Specialized training to avoid exposure is essential for all HD employees. (Sterile hazardous compounding technicians need training in both specialties.) The US Occupational Safety and Health Administration, or OSHA, requires each hospital or pharmacy to establish a hazardous communication standard (HCS) outlining the policies, procedures, and practices to ensure worker safety in all aspects of the manipulation and distribution of these drugs and chemicals for all workers.
The HD training must be documented, and each technician must be assessed for competency prior to any HD compounding and then reassessed at least every 12 months. The training must include at least the following:
overview of entity’s list of HDs and their risks
review of the entity’s SOPs related to handling of HDs
proper use of PPE
proper use of equipment and devices (e.g., engineering controls)
response to known or suspected HD exposure
spill management
proper disposal of HDs and trace-contaminated materials
 Safety Alert
Safety Alert
If you deal with HDs in any capacity—even if not compounding—PPE must be worn!
The hospital or facility must designate a pharmacist to be the compounding supervisor responsible for personnel competency and developing and implementing appropriate HD procedures in compliance with USP Chapter <800> as well as applicable federal and state laws, regulations, and standards.
Protective Clothing
According to USP Chapter <800>, personal protective equipment (PPE)—specialized clothing and gear—must be worn when working with hazardous agents. These guidelines are similar to the ones discussed in Chapter 12 for preparing CSPs, but HD compounding has additional requirements. The PPE must meet the NIOSH standards and include (among other potential equipment) the following:
a non-permeable chemo rated gown
hair cover and two pairs of shoe covers
eye protection (goggles and shields)
two pairs of chemo rated gloves; the top must be sterile when compounding
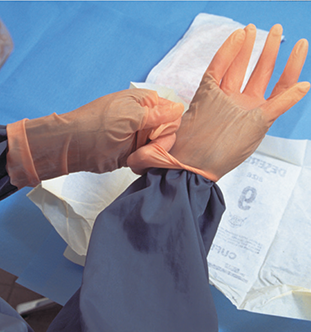
When working with hazardous drug agents, pharmacy technicians must use additional precautions, such as double chemotherapy gloves.
The goggles and face shields are designed to guard compounders’ eyes and faces, as well as breathing passages and lungs, and must particularly be NIOSH approved. The gown must be disposable, lint-free, and impervious to liquids and solids, with a closed front and long stretchy cuffed sleeves to close off airflow. (Cloth laboratory coats, surgical scrubs, or isolation gowns are not appropriate when handling HDs because they permit the permeation of HDs and can hold spilled drugs against the skin and increase exposure. Chemotherapy gowns must be changed according to manufacturer recommendations or every 2–3 hours if no recommendation exists for the chemo-rated gown.)
Simply wearing sterile compounding gloves is not sufficient for hazardous compounding. Two layers of non-permeable chemotherapy gloves that are powder-free and tested by the ASTM (an international standards testing organization) are required. Double gloving should be done after proper hand hygiene per CDC guidelines, as described in Chapter 12. The outer gloves should be changed every 30 minutes or whenever torn or punctured, and must be sterile if sterile compounding.
The hazardous compounding PPE should be worn by a pharmacy technician when handling HDs and performing any of these functions:
receiving intact supplies (remote from the compounding area)
receiving broken or potentially broken supplies
transporting intact supplies or compounded HDs
receiving intact supplies in the compounding area
stocking and inventory control of the compounding area
nonsterile compounding
sterile compounding
collecting and disposing of compounding waste
routine cleaning
managing spills
 Safety Alert
Safety Alert
If a technician is working with hazardous drugs, additional protection such as a second pair of sterile gloves (known as double gloving) is required. A new gown must be worn if you have a spill or splash.
Before leaving the hazardous drug preparation area, all disposable protective garb must be discarded as trace chemotherapy waste in a specially marked container. No gloves, masks, and so on, can be reused (even after a short break), and no contaminated protective clothing can be worn outside the hazardous compounding area.
Secondary Engineering Controls to Contain Hazardous Agents
Hazardous substances and sterile hazardous substances must be compounded away from sterile products in a containment secondary engineering control (C-SEC) area with an exterior venting system. This way the trace hazardous substances do not flow into other CSP compounding areas and the hospital or the rest of the compounding facility.
As explained in Chapter 12, sterile compounding requires a cleanroom facility with two rooms with ISO air quality standards of Class 8 in the anteroom and Class 7 in the buffer room. To achieve these ISO standards, secondary engineering controls with high-efficiency particulate air (HEPA) filters are integrated into the heating, ventilation, and air conditioning systems (HVAC) of the rooms to create unidirectional airflow and air exchanges. For nonhazardous sterile compounding, filtered air is pumped into the buffer room to create positive air pressure and to have it flow out of the cleanroom areas, taking contaminants with it.
Hazardous compounding airflow, however, goes in the reverse pattern of that in the sterile compounding cleanroom. Hazardous compounding areas must provide negative pressure, with the ventilation system suctioning away the air around the compounder and hazardous product into a filtered exhaust system to keep the toxics away from the compounder and the buffer room.
There are two acceptable basic configurations for the C-SEC. In order to get full dating in USP <797>, the C-SEC must be an ISO 7 negative pressure buffer room with appropriate air changes per hour (ACPH) with ISO 7 anteroom. An alternate option is a segregated compounding area with appropriate ACPH, but may only dispense CSPs with a 12 hours beyond-use date. Both room configurations still require the use of a negative pressure primary engineering control and both C-SECs must follow USP <800> standards.
For both sterile and nonsterile HD compounding, a sink must be available outside the buffer area for handwashing as well as for emergency access to water, but at lease one meter away from the entrance to the negative pressure area. An eyewash station within the contained HD compounding area is necessary for removal of hazardous substances from the eyes and skin.
Primary Engineering Controls to Contain Hazardous Agents
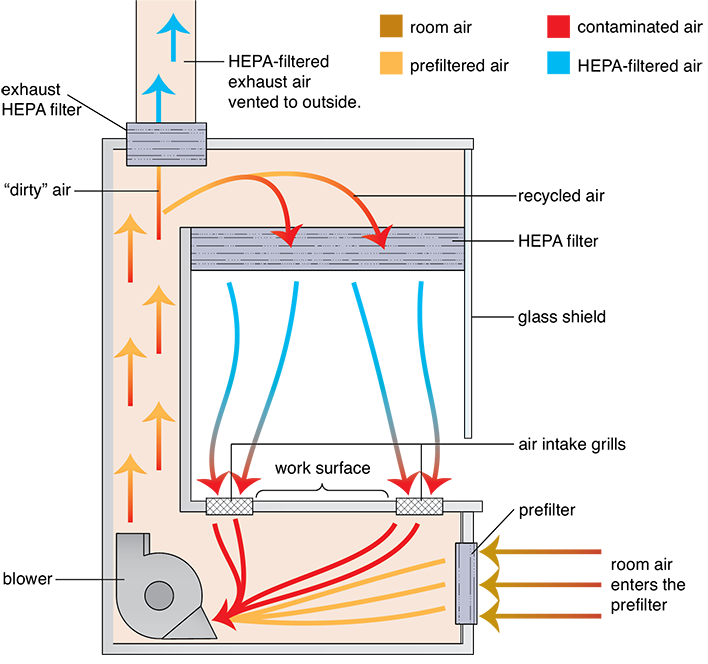
Per USP Chapter <800> standards, all HD compounding must be done in containment primary engineering controls (C-PECs), which provide some level of shielding, enclosed isolation of the DCA, or sealed isolation to contain the HD substances and protect the compounding personnel. These C-PECs must still provide ISO Class 5 air quality in the DCA through the use of a ventilation system with HEPA filtering of the circulating air, air exchanges, and some level of exhausting of the circulated air. The C-PECs, however, have vertical airflow, or downward moving air, instead of the horizontal airflow that occurs in nonhazardous sterile compounding. (See Figure 13.12, which shows how vertical airflow works in one of the types of C-PECs.) The vertical airflow keeps the contaminants from blowing at the person doing the compounding. C-PECs come in various classifications, according to the level of toxicity of the agents compounded within them and the needed protection level to keep the compounder safe from exposure.
Figure 13.12 Side Cutaway of a Class II Biological Safety Cabinet
In a C-PEC with vertical airflow, the room air enters a prefilter and is forced upward through the HEPA filter, then a portion is blown down vertically from the hood onto the DCA to the work surface, where it is sucked back into air intake grills, drawing the air away from the compounder and the outer room. The rest of the air is exhausted through another HEPA filter to an outside space or exterior ventilation system.
Levels of Hazardous Drug Risk and Contained Primary Engineering Controls
NIOSH publishes a list of HDs based on groups of risk. This list is updated every two years and includes a review of existing HDs as well as the addition of newly approved drugs. The NIOSH groups of risk include the following.
Group 1: Antineoplastic drugs. Many of these drugs may also pose a reproductive risk for susceptible populations.
Group 2: Non-antineoplastic drugs that meet one or more of the NIOSH criteria for an HD. Some of these drugs may also pose a reproductive risk for susceptible populations.
Group 3: Drugs that primarily pose a reproductive risk to men and women who are actively trying to conceive and women who are pregnant or breast-feeding (some of these drugs may be present in breast milk).
Table 13.10 General Types of Containment Primary Engineering Controls for Sterile Hazardous Compounding
Each unit has vertical HEPA-filtered airflow, air exchanges, and some level of HEPA-exhausted air, according to USP Chapter <800> (2016) standards. |
Containment Primary Engineering Controls |
Class II biological safety cabinets—provide a containment barrier through a partial or full shield for hazardous compounding protection in various levels
Compounding aseptic isolators—an enclosed PEC with an ingredient transfer station or interchange zone
Sealed sterile compounding isolators—units with completely sealed DCAs, and transfer chamber systems with self-cleaning/gas sterilizing
|
Types of Containment Primary Engineering Controls
Various levels of HEPA-filtered C-PECs are designed to protect the workers and the environment from toxic exposure to the HDs in the aforementioned NIOSH risk groups. Hazardous C-PECs include the many levels of biological safety cabinets (BSCs), the compounding aseptic containment isolator (CACI), and the radio pharmacy isolator used in nuclear pharmacy. These BSCs come in different Class levels of protection and safety to align with the risk level of the substances: Class I, Class II, and Class III. A Class I BSC protects personnel and the environment but does not protect the product/preparation and is not appropriate for sterile compounding. Class II BSCs for compounding come in different levels of filtered venting and recirculated air. Class III is a sealed isolator offering the most protection for the most hazardous compounds.
Table 13.10 shows an overview of the general types of C-PECs. Manufacturers produce variations of the different PEC types, creating hybrid models as they work to continually improve their equipment offerings and keep up with the new USP <800> guidelines. Each HD compounding facility has to train its staff on the ins and outs of its own specific equipment, but there are some standard principles to learn.
Class II Biological Safety Cabinets Class II BSCs are subdivided into even more categories of Class II-A2, B1, and B2 with increased containment at each level. Some C-PECs that are in the Class II-A categories are also known as vertical laminar airflow workbenches (V-LAFWs). Because the airflow is moving downward from the ceiling vent onto the DCA rather than from the back wall, the zone of turbulence is in a different place. The compounding technician must be careful not to block the airflow to the CSP in the DCA.

A technician prepares hazardous drugs in a CACI.
Class II-A2, B1, and B2 have less recirculated air and more filtered venting to the outside of the cleanroom environment for cleaner air exchanges. The Class II-B cabinets also have a lower shield for additional protection, some extending to the counter like a hinged door.
Since the filtered airflow flows down onto the DCA, the key zone of turbulence has changed from a horizontal LAFW. So, the zone exists below any action or supplies. Technicians have to be very careful that they do not put their hands or tools above the CSP in the DCA. They do not want to block air flowing down on the CSP or compounding actions in any way.
Compounding Aseptic Isolators (CAI) The CAI creates a positive pressure clean air controlled environment. Whereas the compounding aseptic containment isolator (CACI) creates a negative pressure clean air controlled environment. Both CAIs and CACIs are types of PECs known as RABS or Restricted Access Barrier Systems. RABS are treated no differently than other primary engineering controls and all personal protective equipment is required when compounding in a RAB. Beyond-use dating is determined by the environments the PEC is placed in and not they type of PEC it is compound in. The CACI, like the compounding aseptic isolators, is an enclosed PEC, with an ingredient transfer station or interchange zone and ingredient transfer stations. As in all the other PECs, the DCA is maintained at ISO Class 5 air quality by the HEPA-filtered ventilation system in the unit. The CACI has heavy-duty NIOSHapproved gloves projecting through it. The hazardous CSP supplies and ingredients are moved inside the DCA via a transfer port (an enclosed movable chamber that is not fully sealed). The RABS shield must be opened to conduct a full deep clean and decontamination. This includes cleaning under the work tray. Daily cleaning and decontamination is more difficult and requires special tools to reach all surfaces of the RAB.
 Safety Alert
Safety Alert
Placing a biological safety cabinet Class II-B in a separate negative-pressure room decreases the risk of employee exposure to dangerous contaminants, which is why it is required.
Class III Biological Safety Cabinets The Class III BSC is like the compounding aseptic isolator in having a completely sealed DCA and sealed supply transfer chamber and a self-cleaning gas and vacuum system, so the unit is not opened by the technician. However, the BSC Class III has vertical airflow and complete filtered external venting with no recirculated air, and some units have robotic arms instead of a DCA for extra protection.
Nuclear Compounding Isolators The radiopharmacy isolators are also completely sealed, though they have lead or tungsten shielding (including leaded glass) to protect the compounding personnel from nuclear exposure. Many of these isolators have computer screens to view the compounding for even greater protection.
Supplemental Engineering Controls for Hazardous Compounding
In hazardous sterile compounding, slightly different processes are used for working with vials and ampules than in general sterile compounding, and a few additional protective supplies should be used: a chemotherapy compounding mat, closed-system transfer devices, and chemotherapy engineering controls. A supplemental engineering control is an adjunct control (e.g., CSTD) that may be used concurrently with primary and secondary engineering controls. Supplemental engineering controls offer additional levels of protection and may facilitate enhanced occupational protection, especially when handling HDs outside of primary and secondary engineering controls such as for nursing during adminstration.

A chemotherapy compounding mat is placed on the work surface before each session to catch toxic contaminants.
Chemotherapy Compounding Mat
A chemotherapy compounding covering for the counter that has o of absorbent material to soak up po spills within the DCA. The other side of the mat is made of a low-permeability material that prevents fluid from seeping through to the work surface. Before beginning each HD compounding session, the mat is laid on the counter, and afterwards the mat is discarded as contaminated waste in a designated container.
Closed-System Transfer Devices
Supplemental engineering controls are needed to reduce trace hazardous substance exposure during the transfer of toxic agents from one container to the next in compounding. For this purpose there are commercially available closed-system transfer devices (or CSTDs). These are hand-manipulated devices that attach to transfer sites of HDs between containers, such as syringes to vials. CSTDs come in different forms and styles. They use double membranes to provide tightly sealed and dry connections that allow the safe withdrawal of fluid from a vial into a syringe and the safe injection of fluid from a syringe into an IVPB or LVP. The closed system also circumvents pressurization issues with vials, thus preventing the escape of toxic vapors. CSTDs should be used as a supplemental control for compounding inside a BSC or CACI when the HD dosage form allows, and must be used for administration when the dosage form allows.
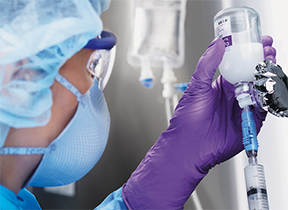
This CSTD (the blue plastic piece connecting the syringe to the bottle) around the vial to the needle-and-syringe site offers the compounder additional protection from vapors within a BSC or a CACI.
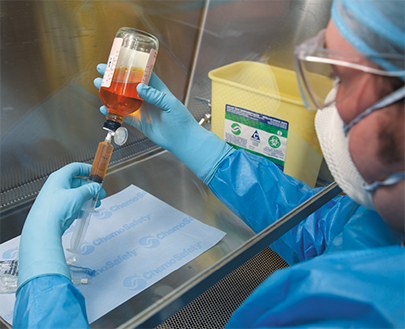
A Class II biological safety cabinet is commonly used to prepare hazardous cancer drugs in the hospital or the infusion pharmacy HD compounding area.
Compounding with Hazardous Agents
When a pharmacy technician is required to prepare a hazardous CSP from a vial medication, a CSTD should be used. If possible, it is recommended to use an injection solution rather than a powder for the active pharmaceutical ingredients.
Occasionally, a pharmacy technician will need to prepare a hazardous CSP from an ampule with an HD. This is done in a manner similar to the general sterile compounding procedures. Some examples of common toxic chemotherapy drugs can be seen in Table 13.11.
Table 13.11 Samples of Common Chemotherapy Drugs
Generic |
Brand |
Indication |
abiraterone |
Zytiga |
Prostate cancer |
bevacizumab |
Avastin |
Colon, lung, renal, ovarian, and brain cancers |
bortezomib |
Velcade |
Multiple myeloma, mantle cell lymphoma |
chlorambucil |
Leukeran |
Chronic lymphocytic leukemia |
cyclophosphamide |
Cytoxan, Neosar, Endoxan, Procytox, Revimmune, Cycloblastin |
Lymphomas, brain cancer, leukemia, prostate cancer |
etoposide |
etoposide |
Lung cancer, testicular cancer |
gemcitabine |
Gemzar |
Pancreatic, lung, bladder, breast, and ovarian cancers, soft tissue sarcoma |
imatinib |
Gleevec |
Leukemia and other cancers of blood cells, gastrointestinal stromal tumors |
irinotecan |
Camptosar, Onivyde (lipsome injection) |
Metastatic colon or rectal cancer |
lenalidomide |
Revlimid |
multiple myeloma |
mercaptopurine |
Purinethol |
Some types of leukemia |
methotrexate |
Trexall, Folex |
Breast, bladder, bone, lung, head and neck, ovarian, cervical, and testicular cancers and others |
pembrolizumab |
Keytruda |
non-small cell lung cancer, classical Hodgkin lymphoma, melanoma, gastric and cervical cancer |
rituximab |
Rituxan |
Leukemias and lymphomas, rheumatoid arthritis |
oxaliplatin |
Eloxatin |
Colon or rectal cancer |
trastuzumab |
Herceptin |
Breast cancer |
Dispensing Hazardous Oral Drugs
Hazardous oral drugs may be handled in community, home health care, long-term care, and hospital pharmacy settings. HDs that are in sealed unit-dose or unit-of-use dose packaging and do not need modification do not require workers to wear any additional protection unless there are manufacturer cautions or compromises in the packaging.
 Safety Alert
Safety Alert
With HD vials, do not inject a volume of air equal to or greater than the volume of drug to be withdrawn.
During routine handling of hazardous tablets and capsules, workers should wear one pair of HD gloves of sufficient quality and thickness, a gown, or any PPE dictated by facility SOPs, according to the faculties’ assessment of risk. Counting and pouring these drugs (such as methotrexate) should be done carefully, and contaminated equipment like counting trays should be immediately cleaned with detergent and/or a decontaminating agent and rinsed after each use. HD tablet and capsule forms of hazardous materials should not be placed in automated counting or packaging machines. When hospital pharmacy technicians repackage a hazardous oral agent into unit-dose bubble packaging, they must wear personal protective equipment. Manipulation of any HDs (such as crushing tablets or opening capsules) should be performed carefully within a C-PEC using appropriate PPE.
Handling Hazardous Drug Stock and Products
Even when receiving, stocking, inventorying, and disposing of HDs, pharmacy technicians must wear double gloves and often other forms of PPE, per hospital and OSHA policies. A spill kit must be readily accessible in the receiving area. A spill kit is a container of supplies, warning signage, and related materials used to contain an HD spill. Its contents and use will be discussed in more detail in the cleaning and disposal of HDs section later in this chapter.
Damaged packages should be inspected in a segregated, isolated area that has negative, neutral, or normal pressure. The receipt of broken vials of HDs should be treated as a hazardous agents spill, and reported to the compounding pharmacist supervisor.
Cleanup should follow established written procedures, as discussed later in this chapter.
Transporting Hazardous Compounded Sterile Products
Personnel who transport HDs to the compounding area, storage, and nursing units also must be trained and their competency documented. HD handling requires safeguards, particularly to minimize the potential for breakage or spill exposure. Technicians should be well versed in how to respond to exposure, breakages, and spills, and should know how to use a spill kit.
Within the hospital, the HDs must be transported in closed protective containers. If the final delivery is outside the hospital, infusion, or compounding pharmacy, the technician must select packing containers and protective materials to maintain physical integrity, stability, and sterility (if needed) of the HDs during transit. All CSPs must be delivered to the nursing unit (or home) in a sealable plastic bag to prevent damage, leakage, contamination, or degradation; this becomes especially important with hazardous CSPs.
Storage
Hazardous drugs should be delivered directly to the storage area upon delivery and immediately inventoried. During this process, the HDs should be separated from other medications to prevent contamination and exposure as well as to reduce the potential error of pulling a look-alike container from an adjacent shelf or bin.
Storage areas, including drug cartons, shelves, bins, counters, and trays, should carry appropriate, brightly colored warning labels (“Caution: Hazardous Agents”) and be designed to maximize product recognition and minimize breakage. For example, storage shelves should have a barrier at the front; carts should have rims; and HDs should be stored at eye level or lower. Ideal storage is in a room with frequent air exchanges and negative air pressure to dilute or remove potential airborne contaminants.
Hazardous drugs requiring refrigeration should be stored separately from other drugs in bins that prevent breakage and that would adequately contain the leakage if it should occur. This dedicated HD refrigerator must be located in the designated HD storage area or in the HD buffer area. Access to storage areas and work areas for hazardous materials should be limited to specified trained personnel. A list of hazardous drugs should be compiled and posted in appropriate locations throughout the workplace.
Labeling
All CSPs containing hazardous agents must be properly labeled. The labels must contain the patient’s name and room number, the solution name and volume, the drug name(s) and dosage, CSP administration information, and storage requirements. Hazardous agents also require additional labeling that clearly identifies the CSP as a hazardous agent.

All CSPs that contain HDs need to be assigned appropriate beyond-use dates. If the HD CSP is prepared and sent off-site, then the medication must be insulated and stored at the appropriate temperature during transit.
Administration of Hazardous Products and Compounded Sterile Products
Besides being clearly labeled as hazardous, all HD CSPs should be primed according to institutional guidelines and USP Chapter <800>. Syringes filled with hazardous agents should never be filled more than full because overly full syringes have a greater potential for the syringe plunger to become separated from the barrel. If the medication is to be dispensed from the syringe, then the HD solution should be cleared from the needle and hub, and the needle should be replaced with a locking syringe cap. The exterior surface of the prepared syringe should be cleaned with a lint-free wipe saturated with sterile 70% IPA and then labeled. If the HD medication is to be added to an IV bag, special care should be used to prevent a puncture of the bag.
 Pharm Fact
Pharm Fact
Technicians prime the IV tubing before they inject the HD into the bag.
Priming an IV Administration Set
As opposed to general CSPs, HD IV administration sets should always be primed in the DCA of the biological safety cabinet to avoid endangering the nurse or patient at the bedside. To prime the IV administration set before adding the HD, the technician should insert the spike of the administration set into the CSP’s tubing port as would have been done by the bedside. This action allows fluid to run from the CSP through the tubing, thus priming the tubing. As with the other CSPs, the chamber, connections, and ports must be tapped to remove air bubbles. The exterior surface of the administration set and the entire bag should then be wiped with sterile 70% IPA and labeled.
Administration
Depending on the specific type of CSP, the nurse may administer the hazardous CSP through the primed IV administration set or a drug-specific closed-system transfer device at the bedside.
To administer the hazardous medication, the nurse must also wear shield (if there is a risk of splashing), chemotherapy gloves, and a chemo gown, like the HD compounding technician. Typically, two nurses check the medication dose and labeling before administering it to the patient. This precautionary measure minimizes the risk of drug administration errors. An incorrect HD dose, especially in a pediatric patient, could prove deadly.
Once the administration of the medication is completed, the nurse disposes of the HD protective garb in a designated hazardous waste container. As with any other medication, the nurse must do the proper documentation.
Cleaning and Decontamination
In general, the standard HD personal protective equipment, including eye and face protection and ASTM chemotherapy gloves, should always be worn for cleaning, disinfecting, decontaminating the C-PECs and CACIs, and deactivating HDs in the compounding area, using appropriate products. Chemical deactivation of an HD should be done with 2% sodium hypochlorite or other appropriate agent. Alcohol is not an effective deactivating or HD cleaning agent.
 Safety Alert
Safety Alert
A technician should never use bare hands to handle hazardous fluids or glass shards. Broken glass should be placed in an appropriate, puncture-proof container.
Decontamination is the inactivation, neutralization, or removal of HD residue from surfaces. This is done with a low-lint wipe, which is then contained and discarded as contaminated waste. HD contamination in a BSC or CACI may be reduced by wiping down HD containers before introducing them, as studies show that the outside of the vials have trace contamination on them when they arrive in the pharmacy. The BSC or CACI should also be decontaminated daily or any time a spill occurs.
Since the HEPA filter is located in the ceiling of the hood in a vertical airflow C-PEC, the pattern of the cleaning strokes and order is different from the horizontal hood. Clean the HEPA filter cover, being careful not to get the HEPA filter wet, then the back wall of the hood, the bar, sides, and the front facing. The main work surface will be the last surface cleaned as it is the dirtiest surface. Use linear, overlapping strokes, following the direction of airflow starting at the top and moving horizontally to the bottom. Use a decontaminating agent, a cleaning agent and then a disinfecting agent like sterile 70% IPA. Keep in mind that some decontaminating agents will require the surface to be wiped twice. The procedures for working with and cleaning PECs that encased hazardous drugs are even more rigorous and are outlined in manufacturer specifications. Though the CACI is enclosed while in use, it also must be opened at least monthly to clean under the work tray and clean with a sporicidal agent. Personnel should also wear respiratory protection whenever they clean under the work tray of the BSC or CACI.
BSCs and CACIs used for compounding HDs need to be disinfected at the beginning of the workday, between batches of compounding medications, at the beginning of each subsequent shift, routinely during compounding, and after any time the units have been powered off. The area under the work tray should be cleaned at least monthly to reduce the contamination level in the BSCs and CACIs. USP <800> also requires that the hood is decontaminated between the compounding of different HDs.
Cleaning a Hazardous Spill
Spills must be contained and cleaned immediately by trained workers. Only trained workers with appropriate PPE should manage an HD spill, and they must post warning signs to restrict access to the spill area. The goal is to ensure that the staff, patients, and visitors are not exposed and that the healthcare environment (both inside and outside the medical facility) is not contaminated.
When cleaning spills, the trained personnel must wear proper attire—including a gown, double gloves, goggles, and a mask or a NIOSH-certified respirator.

Some sharps can be placed in sealed containers and in a sharps disposal container in a C-SEC, if approved of in facility policies. Other HD waste must go in specific hazardous waste disposal containers.
HD spill kits that contain all of the materials needed must be readily available in all areas where HDs are routinely handled. Spill kits contain materials to control, clean up, and decontaminate spills of up to 1,000 mL (see Table 13.12).
Table 13.12 Typical Contents of a Spill Kit
Appendix I—Recommended contents of HD spill kit: |
|
In cleanup, technicians first start by absorbing the bulk of the spill with a large absorbent mat. Then they start from the edge and work inward, using absorbent sheets, spill pads, or pillows for the liquids, and damp cloths or towels for solids. Then they should use spill pads and water to rinse the area, and detergent to remove residue. The spill area should be wiped down with a detergent solution (or with the facility-approved decontaminating agent) at least three times to ensure that the hazardous agent has been removed from the spill area.
Spills within the BSC or the CACI require additional cleaning steps. The drain trough should be thoroughly cleaned and the cabinet decontaminated according to manufacturer specifications. All contaminated materials from a spill should be sealed in hazardous waste containers and placed in leak-resistant containers. The spill, the cleanup, and any personnel exposure must be documented.
Disposal of Hazardous Agents and Supplies
When disposing of all HD waste (including unused and unusable HDs) from the C-SECs, BSCs, CACIs, and spills, technicians must comply with all applicable federal, state, local, and facility regulations. They must be aware of the hospital’s proper procedures for the disposal of contaminated drugs, supplies, and clothing. Direct spill waste must be placed in hazardous waste containers. Supplies that held the HDs—such as vials, ampules, IV bags, needles, straws, and syringes—contain trace hazardous waste, which is why they must be treated as hazardous waste. Similarly, so must all items used in the handling, compounding, dispensing, administration, or disposal of HDs, such as gowns, gloves, goggles, and wipes. The worker should remove contaminated garments and gloves and dispose of them in specially designated hazardous or chemotherapy waste containers. No protective clothing should be taken outside the area where the exposure occurred.
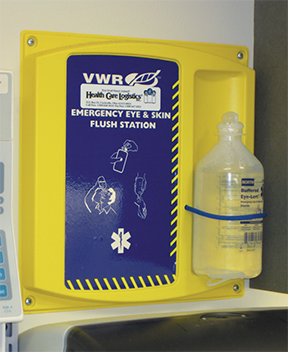
A sink and an eyewash station must be located near to the HD compounding area.
Workers who have direct exposure to HDs during sterile compounding or are contaminated during the spill or spill cleanup, require immediate treatment. Written policies should delineate exact procedures to follow. Every hazardous substance has its Safety Data Sheet filed within the pharmacy department, as explained earlier, outlining specific recommendations on how to treat various exposures.
If the skin is exposed, the worker should use water to flush the affected area immediately and then thoroughly cleanse the area with soap and water. If the substance comes into contact with the eyes, the worker should flush the eyes with large amounts of water or use an eye-flush kit. Workers must also wash their hands thoroughly and be sent or escorted to the emergency room. The accidental exposure should be reported to the supervisor, who should notify other staff members to avoid the spread of contamination. An incident report documenting the spill, exposure, and cleanup must be completed. Follow-up medical surveillance of the exposed worker is required in USP Chapter <800>.
Hazardous Drug Containment Quality Control
To ensure containment of HDs, procedures for containment quality control, such as environmental wipe sampling, should be performed routinely (such as initially as a benchmark and at least every six months, or more often as needed, to verify containment in the C-SEC room) to detect uncontained HD traces. This sampling should include surface wipe sampling of the working area of C-PECs; countertops where finished preparations are placed; areas adjacent to BSCs and CACIs, including the floor directly under the working area; and patient administration areas.